1. Background
Stress causes physiological, histological and behavioral changes. These changes enable the individual to accommodate with stressful situations. Stress causes activation of hypothalamus-pituitary-adrenal and sympathetic systems (1). Also, stress affects some neurotransmitter and hormonal systems. One of the hormones affected by stress is ghrelin. Ghrelin is a growth-hormone-releasing peptide that was discovered for the first time by Kojima and was isolated from rat and human stomachs (2). Ghrelin increases appetite, decreases metabolism and also increases stomach peristalsis. This hormone participates in long-term food intake and there is a strong correlation between body weight and plasma ghrelin level (2). There is controversy regarding the effect of stress on ghrelin secretion. A number of studies have reported an increase (3, 4) while others reported a decrease in secretion (5) of this hormone following a stressful situation. At present, there are a few studies evaluating secretion patterns of ghrelin hormone after chronic immobilization stress in different sexes. In addition, physiological effects of stress depend on a variety of factors including, sex and strain, kind of stress, stress intensity and duration (6-8).
2. Objectives
The aim of this study was to evaluate the effects of chronic immobilization stress on serum ghrelin level, food consumption and body weight in both female and male rats.
3. Materials and Methods
Forty adult (20 male and 20 female) Sprague-Dawley rats (200 - 220 g) were studied. The animals were maintained under standard conditions (temperature 22 ± 2°C, lights on at 6:00 to 18:00 hours) with free access to food and water. All animal experimental procedures were done in accordance with the Jahrom University of Medical Sciences Ethics committee. These animals were divided to four groups, as follows:
Control groups: male and female rats without stress. Experimental groups: stressed male and female rats. Immobilization stress was carried out by placing the animals in a transparent plastic tube made of Plexiglas material. This stress was applied for 120 minutes per day for 14 days. On the fourteenth day, blood sample were directly obtained from the heart. Serums were kept at -60°C until the day of the analysis. Measurement of serum ghrelin level was done with the rat ghrelin ELISA Kit (Biovendor, Check-Slovakia, RD394063400R. lot: x10-110). Body weight was measured on the first and fourteenth day and difference between the two measurements was recorded as weight change. Food consumption was measured daily.
3.1. Statistical Analysis
The SPSS Software version 18 was used for data analysis. Statistical t-tests were performed for the two groups. The values were shown as mean ± SEM. P values of ≤ 0.05 were considered significant.
4. Results
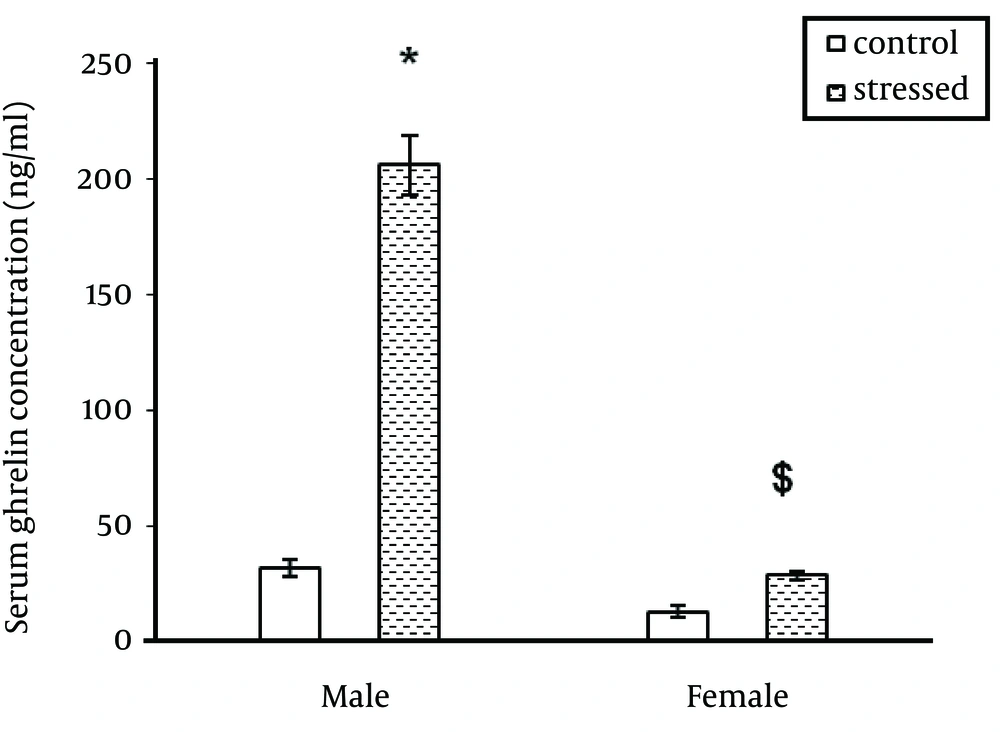
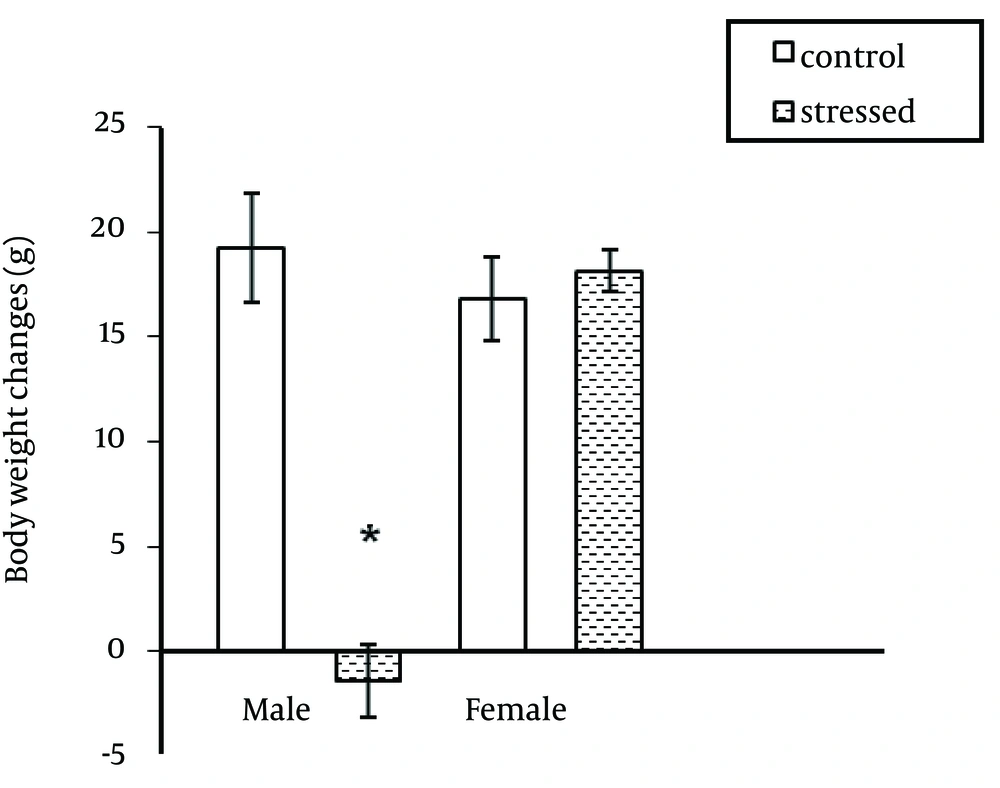
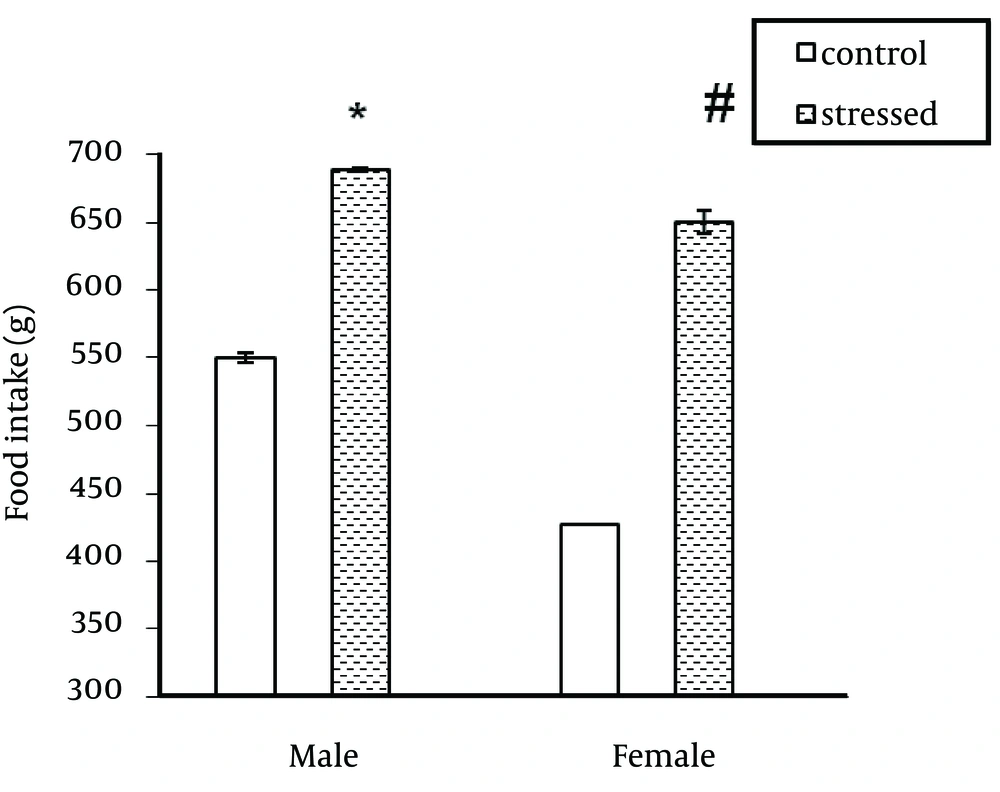
As indicated in Figure 1, stress significantly increased serum ghrelin level in male rats (P < 0.0001) but had no significant effect on serum ghrelin level in female rats. In addition, the ghrelin levels were different between male and female rats in stressed and unstressed conditions. This difference was significant between stressed female and male rats (Figure 1). Immobilization stress significantly decreased body weight in male rats (P ˂ 0.0001). This stress had no significant effect on female body weight (Figure 2). Food consumption significantly (P ˂ 0.0001) increased in stressed female and male rats (Figure 3).
5. Discussion
The results of the present study on the effect of stress on ghrelin level were the same as some previous studies (9-12). Therefore, it seems that stresses including acute or chronic stresses increase ghrelin secretion from the stomach. In addition to its important role on appetite regulation, ghrelin acts as a neuroendocrine transmitter in behavioral responses against stressful factors and decreases stress effects and actually regulates stress (13, 14). On the other hand in this study, ghrelin level was higher in male rats than female rats in stressed and unstressed conditions. It seems that female gonadal hormone (estrogen) is involved in the regulation of ghrelin expression. Previous reports have claimed that ovariectomy increases plasma ghrelin levels in rats (15, 16). In the present study, immobilization stress resulted an increase in food consumption in male and female rats. The results of previous studies about the effect of stress on food consumption have been controversial. A number of studies have reported an increase (17-20) and others reported a decrease in food intake following stress (8, 21). It seems the magnitude of changes in food intake after exposure to stressors is related to stress intensity, sex and rat strain (7, 8). There are also individual differences in stress-induced changes of food intake. According to Macht et al. noise stress resulted no significant change in food intake in two thirds of the stressed rats and decrease in food consumption in one third of stressed rats (21). In addition, there are some reports that show the anti-appetite effects of stress and corticotrophin releasing hormone (CRH) are decreased by repeated stressor or CRH injection (22). Furthermore, ghrelin can increase neuropeptide Y secretion, which stimulates appetite (23). Consistent with a number of previous studies, immobilization stress resulted in a reduction in body weight gain in male rats (24). In this study the effect of immobilization stress on body weight was different between male and female rats. This stress causes weight loss in male rats without significant effect on female body weight. Previous studies indicated that strain and sex of rats is important when evaluating behavioral and physiological responses to stress (25). Faraday (2002), reported that immobilization stress significantly decreased feeding and body weight of males but had no effect on female rats (8). The results of this study show that serum ghrelin concentration is sex dependent. Also, effect of stress on body weight is gender-depended. However, more studies should be performed about the effect of stress on ghrelin level and the relationship between this hormone and gonad hormones in two genders.