1. Context
Military people and athlete are encountered with variety of casualties and injuries during their physical activity in playing ground or in combat situation. Platelet-rich plasma (PRP) is being introduced in skin lesion treatment, facial rejuvenation, and dentistry and recently, its advantages are being employed to other branches of medical science such as orthopedic practices and dermatology. PRP medical application in sport medicine is recognized as a promising method to improve the bone injuries and pain control (1).
Similarity in sport injuries and war casualties makes PRP a potential treatment for military medical implications. Plasma exists in blood and forms 55% of blood volume and water constitutes 93% of plasma. Plasma carries red blood cells (RBCs), platelets, white blood cells (WBCs), ions, proteins, minerals, carbon dioxide, and glucose. In fact, PRP refers to a part of the blood left when the RBCs and WBCs are removed and therefore, plasma remains with a high proportion of growth factors, stem cells, and platelets (2). PRP could be defined also as autologous blood with platelets concentration of 94% while normal platelets concentration is only 6%. Platelets half-life is said to be seven days within the body and the normal count in the blood is 150 × 109/L to 350 × 109/L (3). In fact, PRP is the serum embodying thrombocytes; platelets have no nucleus, and are comprised of many elements including 1000 proteins acting as pulses and located inside or outside of the membrane. They contain small granules known as alpha, delta, and lambda and particularly 50 to 80 alpha granules per platelet. In those granules almost 30 types of proteins, called growth factors, are present and growth factors are mostly involved in homeostasis and healing process (4). Growth factors are released from platelets through a physiologic activation by human thrombin after the injury, and then are renewed through a cascade process on a regular basis. PRP secretes various growth factors including vascular endothelial growth factor, platelet-derived growth factor (agitate angiogenesis), insulin-like growth factor, and fibroblast growth factor. Platelets gained attention as a promising tool for regenerative medicine following by extensive experiments in oral and maxillofacial surgery (5). Addition of calcium chloride and thrombin to the platelets concentration leads to the active secretion of growth factors from alpha granules.
Some studies indicate that platelets have anti-inflammatory and analgesic effects and secrete antimicrobial peptides and therefore, have antibiotic effects (6). Grows factors secreted in this matrix affect various cell types such as chondrocytes (7), osteoblasts (8), fibroblasts (9), endothelial cells mesenchymal stem cells from different origins (10), myocytes, and tendon cells (11), which make a broad range of surgical and clinical applications and treatments possible (12). Platelets were first described by German anatomist Max Schultz in the mid-1800s. PRP has been an interesting topic in regenerative medicine since the beginning of 1970s (13). PRP was initially used in 1987 in an open heart surgery procedure (14). In the early 1990s, multiple experiments in maxillofacial surgery, skin grafting, and dentistry showed improvement in healing quality with PRP. Since then physicians have applied PRP to boost bone healing after spinal injury and soft tissue recovery caused by plastic surgery (15, 16). In fact, history of PRP started much earlier with the research works about the fibrin glues used to improve skin wound healing in a rat model in 1970 (17, 18).
Plastic surgeons and oral surgeons were among the first group of experts who applied the PRP in their surgical operations and then observed improved recovery level (19). In an animal model in 1982, a rabbit cornea model was investigated and showed that platelets along with fibrin trigger a process compulsory for tissue lesion to repair including collagen synthesis, cell migration, angiogenesis, and fibroplasias (4). At the time, PRP therapy was an expensive technique, but various scientists began to implement it on joints injuries, then it was tried by the professional athletes to cure their injuries. In the early years of 2000s, the PRP was use in orthopedics to boost healing in fractures and bone grafts and then sports medicine widely used PRP for soft tissue repair (20). In 2006 Mishra and Pavelko, published the first human study of the use of PRP for chronic tendon problems (21).
1.1. Classification of Platelet Productions
According to the role and different components of PRP derivatives, PRP preparations are classified into four major groups, which are separated based on the quantity of present cells, especially leukocytes and fibrin structure. Four PRP major families are as bellows:
1. Pure PRP or leukocyte poor PRP; these preparations not only include no leukocytes but also have low density of fibrin network. The products of this category are used in gel form or liquid solutions, which are mainly used in sport medicine injuries.
2. Leukocyte PRP; L-PRP preparations contain leukocytes and low-density fibrin network. The preparations of this family are widely used in general surgery, orthopedics, and sports medicine.
3. Pure platelet rich fibrin or leukocyte poor platelet rich fibrin includes no leukocytes, but contains high-density fibrin network.
4. Leukocyte and platelet rich fibrin are productions with leukocytes and high-density fibrin that only exist in the form of strong activated gel.
The above classification system was proposed in 2009, then argued and accepted in a multi-disciplinary conference in 2012; therefore, discussed terminology and classification are cited in many fields, mainly in oral and maxillofacial surgeries (3, 5).
1.2. Physiological Mechanism of Platelet Rich Plasma
Secretion of growth factors begins within ten minutes of activation process then continues release of growth factors into the tissue occurs and survival of the platelets lasts for seven days. The influx of macrophages and stem cells would be stimulated during the activation and the initial platelets concentration indicates the time of wound healing (22). Platelets are formed by fragmentation of megakaryocytes in bone marrow and help the formation of blood clots; life span of platelets is eight to 12 days (23). PRP injections intend to trigger the inflammatory response, which promotes the healing process by renovating injured tissue structure and simultaneously preventing further tissue degeneration (24). Activated platelets signal to distant repair cells, including adult stem cells, to approach to the injured tissue. Rising in the number of platelets increases the future influx of stem and repair cells and the main existing three plasma proteins, ie, fibronectin, fibrin, and vitronectin, which cooperate to form a repair matrix (25).
Because of autologous nature of PRP, it is safe regarding transmission of blood-borne diseases, eg, AIDS and hepatitis, or rejection of blood at the time of stimulation of tissue regeneration, even during each phase of tissue repairing process. PRP preparation requires approximately 15 minutes and the final product is then ready for injection under ultrasonic assistance. In general, PRP would be produced by two basic methods including centrifugation of whole blood and then separation of the plasma layer and extraction of the buffy coat layer. Preparations of buffy coat are aimed to keep the highest number of platelets; therefore, it contains maximum concentration of leukocytes and erythrocytes. In order to generate buffy coat preparations, long duration along with high centrifuge spin rates are used, whereas plasma-based products are obtained using a slower rate in a shorter period. Therefore, plasma-based PRPs include fewer platelets (26, 27). PRP is also produced using spinning kits, but viscosity, volume, and presence of blood cells vary based on the kits on the market (28).
2. Evidence Acquisition
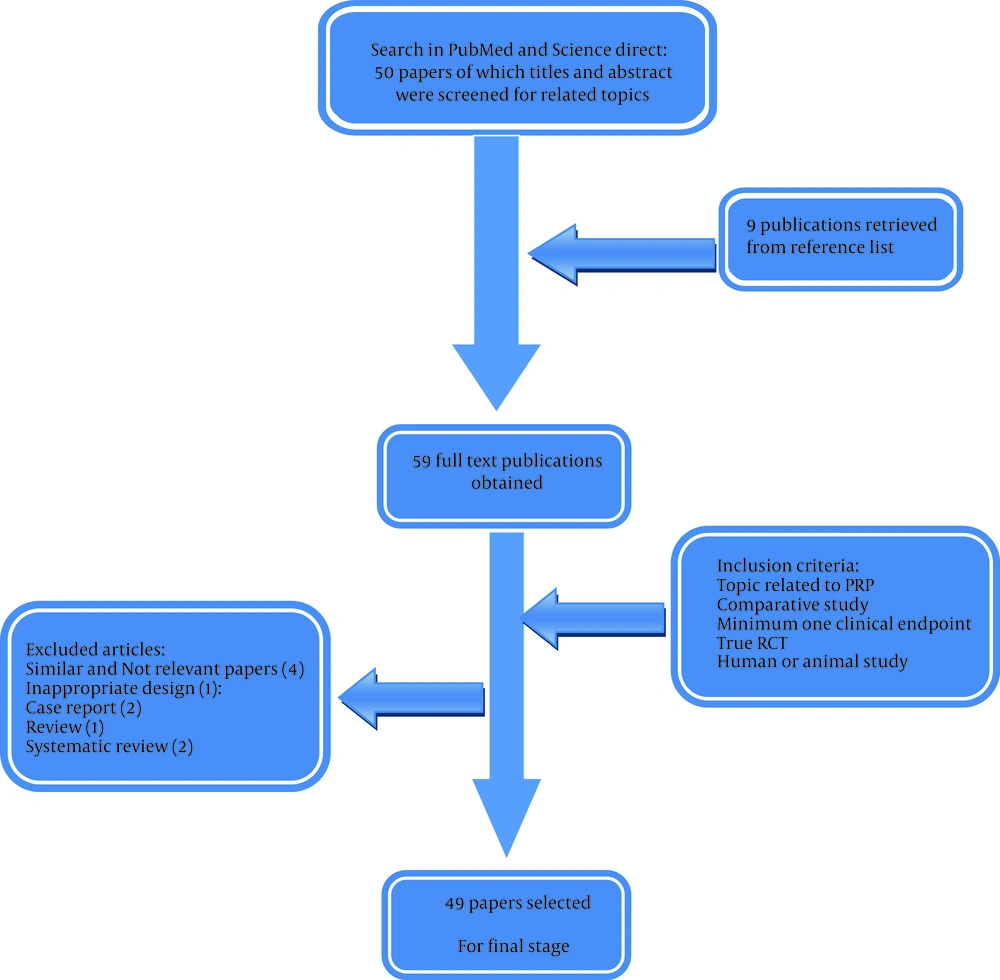
The search for potentially relevant studies was performed in PubMed, Science Direct, and Wiley for all publications up to January 15, 2015. The primary search of the literature was done by one investigator (Farzad N) under administration of the principal investigator (Farshad N), who is a PRP therapy expert. After controlling the title and abstract of papers non-relevant trials were excluded and appraisal of eligibility assessment for the remaining studies was performed carefully. The subsequent medical MeSH terms were used: “PRP” and “musculoskeletal injuries”. No limitations on publication status or date were made. Several language publications were found, but only papers written in English language were included. In addition, reference lists of relevant trials were searched for other potentially appropriate publications. In order to assess the perspective of PRP application in military medicine, relevant studies with regenerative medicine approach were retrieved, which they were mainly in the field of wound healing, tendon injury, and bone grafting. Twenty-six papers were retrieved from PubMed Library and 33 papers from Science Direct and Wiley online. The similar and unrelated papers were deleted from the study and 55 papers were remained. Based on eligibility criteria by authors, 49 papers were approved for the study. In this review, inclusion criteria were based on the outcomes such as success in PRP therapy, and the preferred studies were based on evidence-based medicine, which were experimented on the animals and humans (Figure 1).
2.1. Inclusion Criteria
Appropriate studies needed to report on topics related to musculoskeletal injuries and PRP, and needed to report at least one clinical endpoint. This systematic review only included (i) randomized clinical trials (RCTs), (ii) group studies regarding humans or animals, and (iii) comparative studies.
2.2. Data Extraction
Content from the selected studies were reviewed according to topic, human or animal studies, and outcomes.
3. Results
From 49 papers selected for reviewing, some papers discussed cell-based models, some were about animal models, and some were RCTs in tendon injuries, bone injuries, and skin lesions. Results from evaluated studies were analyzed and reviewed by authors in six subcategories including bone injury, tendon injury, dermatology, alopecia, wound healing, rejuvenation, and hair growth. According to reviewed papers PRP therapy is a relatively simple nonsurgical treatment for many fields including sports medicine, orthopedics, cosmetics, faciomaxillary, and urology, and the aim is to increase the healing properties of the organ by increasing the amount of growth factors. There are promising results about the use of PRP in soft tissue injuries, oral surgery, skin wound healing, spinal surgery, trauma surgery, burns and plastic surgery, gastrointestinal surgeries, heart bypass surgery, and joint injuries and arthritis (29). One of the most interesting applications of PRP is in dermatology especially in skin rejuvenation (30). PRP was acknowledged by the popular media after its extensive use in treating sports injuries in professional athletes (31).
3.1. Bone Injury
Due to the presence of large amounts of growth factors and cytokines, higher bone augmentation and higher bone density is observed by injecting PRP. PRP use in healing mucosal wounds showed a significant raise in capillaries and speeding the wound healing. Mixture of PRP and gelatin hydrogel showed synergism in bone regeneration process and in vitro mixing of PRP with mesenchymal stem cells was experimented and considerably beneficial effects were reported (32). In bone graft and reconstructive surgery, the application of the different types of PRP such as pure PRP has been investigated and the treatment of peri-implant bone defects during sinus lift procedures and various complex implants were well documented (33). In fact, the presence of bioactive substances in PRP could be a logical explanation for the interesting effects of PRP on bone grafts, which stimulates the production of mesenchymal stem cells and chondrocytes, and finally, improvement in bone regeneration.
3.2. Tendon Injury
Special cells called tenocytes, fibrous collagen protein, and water form tendons. In order to make a strong durable structure that naturally anchors to bone, these proteins weave together (34). Tendons transfer great force and thus, may be injured if they are overused and in case of injury, microtears start to form in the collagen (35, 36). Because of absence of good blood supply, tendons tend to heal slower than other soft tissues and there is only a minimal inflammatory response (37). In an experiment on tendon inflammatory response, experimenting both culture and animal models, the effect of PRP on tendon stem cells was investigated and the anti-inflammatory effects of PRP revealed that PRP led to cell differentiation into active tenocytes and production of abundant collagen (38, 39).
3.3. Dermatology
The use of PRP in dermatology is based on the fact that platelets contain many growth factors in alpha granules. Growth factors have a well-documented role in the process of soft tissue regeneration. In injured tissues, the concentration of growth factors could be beneficial to providing more agility to the regeneration processes (40). In dermatology, collagen repair normally includes three phase: inflammation, proliferation, and remodeling. PRP is used on a surgical or wounded site to stimulate and accelerate healing process. The coagulation of blood in all wounds form a platelet clot and formation of fibrin matrix is the first step of the natural healing process (41, 42). The concept behind improvement of the healing process eventually evolved to a more sophisticated idea of tissue regeneration induced by the growth factors secreted from platelet cells in these PRP preparations. Therefore, PRP can be considered as a new strategy in the field of regenerative medicine (43). The application of PRP in the treatment of alopecia has been investigated with renewed interest in recent years. Some studies seek to establish the molecular mechanisms through which such patients could benefit from PRP. In an in vitro and in vivo study in animals, a greater proliferation of dermal papilla cells was observed when they were incubated with PRP, as compared to controls (44). The study showed that the observed molecular mechanisms emphasize the importance of further investigations into the use of PRP for alopecia. Many experiments were not successful to deliver decisive evidence of the PRP therapy benefits in Dermatology, and RCTs are very rare in dermatology (45).
3.4. Wound Healing
Beside the clinical application, more fundamental studies and experiments were conducted to realize the biologic mechanism of the wound healing. This insight created new hopes about biotechnological composites including scaffolds and growth factors. A key study was conducted in 1996 about the association between fibrin, mesenchymal cells, and growth factors to investigate the process of granulation tissue, which reported that formation of the soft tissue is considerably improved in presence of platelet releasate. The PRP triggers constant stimulation for fibrin to interact with mesenchymal cells and an enhanced development of granulation tissue was demonstrated while slower formation of the granulation tissue was observed in lack of such stimulation. Another experiment in 1997 provided interesting outcomes as follows: fibroblasts are transmitted in proximity of injured tissue by soluble factors; and platelet concentration contains high density of chemotactic factors that use fibroblast in a dose-dependent manner (42). Fibroblast travels next to a rich matrix comprises of fibrin accompanied by fibronectin. The use of PRP to accelerate the healing of wounds inspires the greatest number of clinical studies. PRP has been used in the treatment of chronic wounds in diabetic patients, in the assessment of the speed of re-epithelialization of donor sites in skin grafting. Injection of PRP led to a shorter hospital stay. In addition, a considerable lower amputation rate and less pain were reported in case of PRP application (46). Histopathologic experiments revealed that application of PRP products led to an enhanced neovascularization and also significant improvement in tissue regeneration, which is a key element in wound healing. Two reviews on RTCs concerning the use of PRP in the treatment of chronic wounds resulted in successful outcomes. After reviewing clinical trials, they concluded that the use of PRP could be helpful as an adjuvant agent in the healing of chronic ulcers (47). Unfortunately, there are few reports on the association of laser treatments and a concomitant application of PRP. The group treated with the combination of laser and PRP showed less erythematic and greater subjective satisfaction. The result is not an approved superiority of the combined approach as compared to the isolated application of fractional laser. All wounds leave scars, unless they are very small or superficial. Skin scars have a unique effect on patients’ lives; therefore, many treatments have been proposed for cosmetic and functional improvement of scars. Combination of PRP, carbon dioxide laser, and autologous fat graft led to an impressive therapeutic result for atrophic and contractile scars (48).
3.5. Rejuvenation and Hair Growth
There are few published clinical studies regarding the use of PRP for rejuvenation purposes and most of them neither clearly described the method for obtaining PRP products nor clarified the content of the material obtained. The application of PRP for hair loss treatment will not replace the hair graft, but may cause synergism in association with conventional methods. The PRP led to a significant increase in hair density and sped the growth rate when follicular units were treated with growth factors prior to their implantation; moreover, it seems that PRP delays the anagen phase of the hair growth cycle (49).
4. Conclusions
Despite the multiple studies on the PRP, the results are still contradictory and the published data are difficult to sort and interpret; however, because PRP functions as a medium of cytokines and GFs, this blood product is highly practical for application in numerous conditions in reconstructive and plastic surgery. The complicated interaction of various factors and physiologic mechanisms involved in tissue regeneration makes the application of PRP more remarkable than the use of a single recombinant growth factor. In this review, we reported a valuable effect of PRP on wound healing, which caused improved proliferation of vascularization and endothelial cells. In addition, beneficial effects were also observed when PRP was frequently applied to the wound site. When PRP was applied, not only more wounds healed, but also healing time got noticeably shorter leading to a reduction of sickness-related health expenditures. In fact, PRP can be used in wound care process as an adjuvant agent to promote wound healing, along with conventional techniques. Clinical successes of PRP are combined results of the injury state, severity and duration, patient’s age and PRP characteristics. We believe that the type of PRP plays a key role that has strong effect over the clinical outcome. The different methods utilized for PRP production might be a logical explanation for the uncertain results observed in some clinical studies. The results observed in treating multiple tissue lesions in some clinical conditions are strongly impressive and make PRP therapy a potent treatment for future; however, there is a lack in defining and validating methods, products, clinical indications, and quality parameters of PRP products. This review suggests that PRP brings benefits for different indications within the field of reconstructive and cosmetic surgery. Many conclusive studies support the application of PRP to boost healing quality of diabetic lower limb ulcers and to improve bone grafting. Regarding wound healing and fat grafting, there are several medical publications supporting the PRP application. In theory, the combination of PRP with fat grafts may result in an enhanced survival rate. However, strong evidence from well-designed clinical trials to support PRP therapy for hair growth and skin regeneration is still insufficient. It is clear that PRP is currently in a transition period in regenerative medicine. Hence, at this time, PRP requires more investigation with better scientific studies to assess the efficacy of PRP with high quality RCTs using similar assessment tools. Nevertheless, the implementation of PRP as a clinical alternative would become difficult because its therapeutic use has not evolved toward the standardization of the techniques yet. Insufficient description of the adopted procedures reduces the chance of extensive medical recognition of PRP. We strongly believe that due to the absence of high-quality RTCs and lack of standard protocols for the effective platelet concentration and preparation method of PRP, the PRP efficacy could not be recognized scientifically. Therefore, it is required to create standard criteria for obtaining a PRP of high quality, as well as indicate the proper concentration of platelets for the different clinical conditions. It seems that clinical use of PRP produced “promising” but “inconsistent” results in early trials. PRP therapy could not be considered as a “quick fix” solution in dermatology because collagen regeneration takes four to six months and may need multiple injections therefore PRP therapy is expected to promote long-term healing of the injured tissues.
In this review, we have discussed several beneficial effects of PRP and attempted to discuss and clarify the major mechanisms. Although we consider PRP therapy highly potent, in our point of view, military medicine needs more evidence-based trials and concrete and practical solutions instead of hypothetical benefits to utilize PRP in military medical applications in near future.