1. Background
In the food industry, the biological activity of plant and lichen extracts has been investigated for the possible use of essential oils for the control of pathogenic bacteria. This property is entirely based on the presentation of various biological components including steroidal glycosides, polyhydroxylated sterols, naphthoquinone pigments, and antibacterial peptides (1, 2).
Lichens are a result of a specific symbiotic relation between algal and fungal partners. They synthesize various secondary metabolites called “lichen components”, mostly from the fungal metabolism (3). To date, approximately 350 lichen substances have been known and about 200 have been characterized (4), which are extracellular products from modest molecular weights, crystallized on the hyphal cell walls (5). They make even more than 30% of the dry mass of the thallus (6). Some lichens and their phenolic components have various biological properties such as antiviral, antitumor, anti-inflammatory, analgesic, antipyretic, and antiprotozoal (7-10). Several lichen species have distinguished antibacterial and antifungal properties and they have been used in traditional medicine (7, 11-13). Based on topographical, ecological and floristic criteria, Ilam province in Iran with 160 lichen species is divided into two main zones, each of which is divided into four parts as follows: 1- the Nubo-Sindian zone, including: a) desert, b) stabilized dunes, c) river beds, and d) shrublands, hills, and badlands; 2- the Kurdo-Zagrosian zone, including: a) degraded Quercus forest, mostly cultivated by indigenous people, b) rocky, calcareous and deep slopes bearing the Quercus forest vegetation, c) shrubby parts above the Quercus tree-line, and d) high mountains with low-lying vegetation (14-20).
2. Objectives
The aim of this study was the assessment of in vitro antibacterial and antifungal activities of different lichen extracts against specific microorganisms that cause diseases in humans, animals and plants. No reports are available on antibacterial properties of the chosen lichens from Iran (Iranian lichens) and most of the samples collected from Ilam province (part 2 as Kurdo-Zagrosian zone) were investigated.
3. Materials and Methods
3.1. Lichen Samples
This study was based on non-gelatinose Ramalina farinacea samples previously collected from Mazandaran province, Iran (20). Other specimens collected from Ilam province during summer 2011 were identified as Lecanora prophetae-eliae, Acarospora strigata, Physconia distorta, Placidium squamulosum (non-gelatinose lichens), and Collema crispum (gelatinose lichen) in the previous study (21).
3.2. Microorganisms
Seven bacterial strains including Escherichia coli ATCC1652, Staphylococcus aureus ATCC1885, Enterococcus faecalis ATCC2321, Salmonella typhi ATCC1679, Staphylococcus epidermidis ATCC2405, Bacillus cereus ATCC13061, and Proteus mirabilis ATCC2601 were procured from the mycological collection maintained in the mycological laboratory inside the department of microbiology, Tehran university of medical sciences. The fungi used as test microorganisms (Fusarium moniliforme and Verticillium dahlia) were obtained from the Department of Plant Pathology, Faculty of Agriculture, Tarbiat Modares University, Tehran.
3.3. Preparation of Lichen Extracts
The air-dried powders of lichens (50 g) were extracted in the form of water, acetone, and methanol (250 mL) using Soxhlet extractor. The extracts were filtered by Whatman filter paper (No. 1) and then concentrated under reduced pressure in a rotary evaporator. The obtained residues were stored at -18°C until used in the tests. Then, they were dissolved in 5% (v/v) dimethyl sulfoxide.
3.4. Screening of Antimicrobial Activity
The suspensions of fungal spores were prepared from fresh mature cultures (3 - 7-day-old) grown at 30°C on a potato dextrose agar (PDA) substrate. Spores were rinsed with sterile distilled water, used to determine turbidity spectrophotometrically at 530 nm, and further diluted to approximately 106 CFU/mL (CFU: colony-forming units) according to the procedure recommended by the national committee for clinical laboratory standards (NCCLS) (M 38 - P, 1998). Bacterial inoculi were obtained from bacterial cultures incubated for 24 hours at 37°C on Muller-Hinton agar substrate and brought up by dilution according to the 0.5 McFarland standards to approximately 108 CFU/mL.
Muller-Hinton agar (for bacteria) or sabouraud dextrose agar (for fungi) was seeded with the appropriate inoculum. Being soaked with 2.5 - 37 mg/mL of lichen extract, paper discs (6 mm) were laid on the inoculated substrate. Antibacterial and antifungal activities were determined via standard discdiffusion method [Kosanic and Rankovic (22)], by assaying the diameter of the inhibition zone around the disc. Streptomycin in the case of bacteria and ketoconazole (10 µg/mL) in the case of fungi were used as positive controls. Sterile distilled dimethyl sulfoxide (DMSO) was used as negative control. Determination of the minimal inhibitory concentration (MIC) was done by broth tube dilution method (63 - 1000 mg/mL). All the experiments were performed in triplicate.
3.5. Statistical Analysis
All the statistical analyses were performed using SPSS version 13 statistical software package. The data were analyzed by one-way analysis of variance (ANOVA). P values < 0.01 were considered statistically significant. All the results were expressed as mean ± SD for three experiments in each.
4. Results
The methanol extract of P. squamulosum was effective in all tested Gram-positive and Gram-negative bacteria. The higher zone of inhibition was associated with B. cereus ATCC13061 in 1000 mg/mL concentration. The methanol extract of C. crispum inhibited four bacteria including E. coli ATCC1652, S. aureus ATCC1885, B. cereus ATCC13061, and E. faecalis ATCC2321 (Table 1).
| Bacteria | Lichens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. crispum | P. squamulosum | L. prophetae-eliae | A. strigata | P. biziana | R. farinacea | |||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S. aureus ATCC1885 | 1000 | 1000 | 500 | 1000 | 125 | 250 | n | n | n | n | n | n |
| S. epidermis ATCC2405 | n b | n | 250 | 500 | 125 | 250 | n | n | n | n | n | n |
| E. faecalis ATCC2321 | 1000 | 1000 | 500 | 1000 | 500 | 500 | n | n | n | n | n | n |
| E. coli ATCC1652 | 1000 | 1000 | 500 | 1000 | 250 | 150 | n | n | n | n | n | n |
| S. typhi ATCC1679 | n | n | 250 | 500 | n | n | n | n | n | n | n | n |
| P. mirabilis ATCC2601 | n | n | 250 | 500 | n | n | n | n | n | n | n | n |
| B. cereus ATCC13061 | 1000 | 1000 | 1000 | 1000 | 250 | 250 | n | n | n | n | n | n |
aAbbreviations: MBC, minimum bacterial concentration; MIC, minimum inhibitory concentration.
bn, inactive.
Methanol extracts of A. strigata, P. distorta and R. farinacea besides aqueous extracts of all the tested lichens were inactive against all the tested microorganisms. The acetone extract of L. prophetae-eliae showed antibacterial activity against S. epidermidis ATCC2405 and B. cereus ATCC13061 (Table 2).
aAbsorbed extract by discs in different concentrations (mg/mL).
bAverage diameter of the inhibition zone of the acetone extract of gelatinose and non-gelatinose lichens.
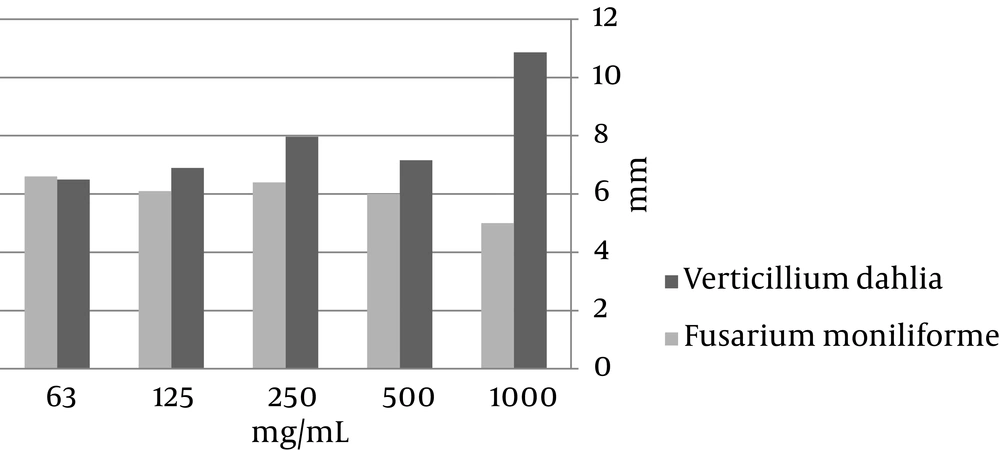
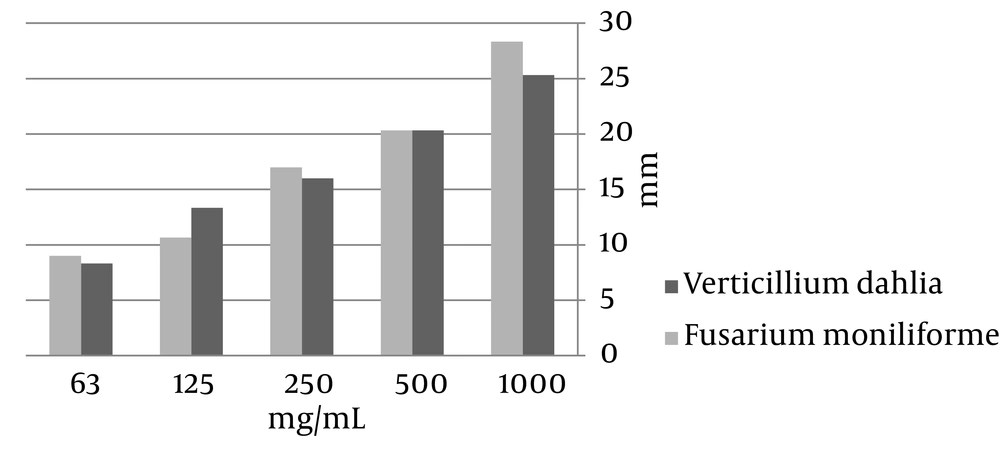
The methanol extracts of C. crispum and P. squamulosum showed antifungal properties, but C. crispum was more effective than P. squamulosum and the effect of P. squamulosum against F. moniliforme was not significant (Figures 1 and 2).
5. Discussion
The results of this study showed that aqueous extractions had no antimicrobial activity and this was probably because the active components produced by lichens were a little diluted (20). Methanol extracts of P. squamulosum showed a high antibacterial activity against all seven tested bacteria. Acetone extract of the species did not show any antibacterial activity. Among the fungi, the most sensitive one against the methanol extract of C. crispum was F. moniliforme (with zones of inhibition ranging from 8 to 20 mm).
There is no report concerning the antibacterial properties of P. squamulosum, L. prophetae-eliae, and C. crispum in the world and in this study, the methanol extracts of these species showed high antibacterial activities and further study for the detection of secondary lichen products is needed.
Bacteria are more sensitive against antibiotics than fungi and it might be related to the different transparency of the cell wall (23). Gram-positive cell wall consists of peptidoglycan (murein) and teichoic acids and Gram-negative cells consist of lipopolysaccharides and lipopolyproteins (24, 25), whereas the hyphal cell wall of fungi consists of polysaccharides such as hitchin and glucan (23, 26). Similar results were reported by Shyam Kumar et al. for different extractions from the C. auriforme (27). Kosanic et al. (26) found an antibacterial activity for the extractions of the C. cristatum. In a study by Jeon et al. A. strigata showed inhibition of more than 80% in mycelial growth of a pathogen (28).
According to the results of this study, the methanol extracts of the tested lichens had significant antimicrobial effects on some microorganisms. Therefore, it can be beneficial in treatment of some diseases caused by tested microorganisms; especially, in terms of drug resistance they can be proper substitutes, but further studies are suggested.