1. Background
Low back pain (LBP) is very common in outpatient and inpatient departments, with an expected 61 Million visits in 2007, from an expected 15 million visits in 1990 (1-3). Low back pain is connected with huge comorbidity and direct social insurance costs because of expanded medicinal services use (4). The financial weight of low back pain is evaluated as $20 and $50 billion yearly in the US economy (5). Also, low back pain results in an expected 149 million lost workdays every year and an extra $28 billion in efficiency misfortunes (6). In spite of the relative pervasiveness of low back pain, and the tremendous money spent on the social insurance framework, chronic low back pain remains a troublesome condition to treat (7, 8). Distinguishing proof of adequate noninvasive, non-pharmacologic treatments could yield significant pick up and bring about considerable populace change in quality of life and expenses associated with LBP. A clinical trial is expected to decide treatment viability of home exercise treatment that is economical, noninvasive and non-pharmacologic.
Many different treatments have been described for chronic LBP, yet the mass of standard randomized trials showing good results for many commonly used treatments is not enough (9-11). A recent systematic review showed that only 47 of 148 treatment methods for chronic LBP have standard randomized trials as the basis of treatment for low back pain (8). Thus, almost all of the popularly used treatments have inadequate or no evidence of effectiveness.
Specific to exercise modalities, multiple exercises are used for treatment of chronic pain conditions, including nonspecific low back pain. A systematic review of exercise literature identified a modest number of moderate and low quality trials that showed evidence of exercise efficacy for selected patients with chronic low back pain, yet there is still no consensus about effectiveness of exercise for treating chronic LBP (8, 12-20). Additionally, reviews have found insufficient quality evidence for recommending for or against the use of exercise therapy for treating chronic low back pain. Similarly, although there is strong personal support, there are no reported quality randomized controlled trials demonstrating efficacy of the exercise for symptomatic relief of chronic low back pain. Regardless of the evidence of efficacy of exercise in treating chronic LBP, it is a common intervention for treating LBP due to high demand for noninvasive, non-pharmacologic interventions. Exercise modalities are highly prescribed due to low cost and low occurrence of side effects (21). Identification of exercise treatments with quality evidence on effectiveness, low cost, and low side effects would be expected to result in improved patient function, reduced pain, reduced morbidity, improved productivity and overall lower health care costs. In this study, we evaluated an exercise therapy method from home exercise category named “Farshad exercise”
Our home exercise (Farshad exercise) is an exercise treatment method. The essential distinction between our exercise and different exercises is its simplicity and inexpensiveness. All required device is a single sofa. Patient lies supine with relaxed arms and body in front of the sofa with a pad, like infant pads, under the head, flexing knees and hips to reach back of the thighs to front side of the sofa and back of the legs to sitting side of the sofa, then relaxes all the body as relaxed as possible, then shakes his/her legs slowly and continuously for 30 minutes. The mechanism(s) of action for this exercise is relaxing spinal and all related muscles. While the exact mechanism of LBP treatment is unknown there are multiple mechanisms, including the one referenced above that may demonstrate efficacy for treating LBP.
2. Objectives
The goal of this trial was to execute a high-quality controlled clinical trial that analyzes the adequacy of the Farshad exercise with a control group (22). A well-designed, high-quality trial provided definable level of evidence for treatment efficacy, and provided a basis for evidence-based recommendation for or against utilization of the Farshad exercise. The outcomes for Farshad exercise, was positive, could significantly impact morbidity by providing a non-invasive, non-pharmacologic treatment for symptomatic relief, and reduced overall disability and health care costs associated with chronic low back pain.
3. Methods
3.1. Participants
Subjects were recruited from a military medical center in Tehran. Enlistment and treatment was conducted at the clinical/research center offices of the AUMS.
Potential study subjects were distinguished through the Institutional Survey Board (ISB) and approved study materials were screened taking into account inclusion and exclusion criteria. In the event that all criteria were met, they booked for consent, and started treatment.
Inclusion criteria for study qualification included chronic low back pain (≥ 3 months term), no emanating pain underneath the knee, ≥ 75% back or buttock pain rather than lower extremity pain, and ready to finish and endure treatment for the study time frame.
Exclusion criteria included earlier history of spinal fusion or failed spinal surgery syndrome, laminectomy, laminotomy or discectomy within 12 months of enlistment, and indicative or interventional infusions or any low back surgeries not said above, including radiofrequency neuroablation inside six months of enlistment. Patients utilizing individual home based electrical stimulation gadgets were avoided. Patients with other concomitant disease (e.g. malignancy, osteoporosis, etc.), which in the assessment of the investigator would block effective patient participation, were also avoided. Patients with active psychiatric issues were also excluded (e.g. utilization of antipsychotic drugs, bipolar disorder and schizophrenia). Patients determined to have a history of significant mood disorder were avoided (e.g. use of antipsychotic medication, bipolar disorder and schizophrenia). Patients who were pregnant at the first visit became pregnant later were excluded.
Consent was acquired before start of treatment. Study subjects gave a copy of the consent record and the report was outlined in detail by a research colleague. Study subjects offered chances to make questions prior to signing the consent form and selection in the study.
3.2. Allocation Procedures
Subjects were allocated after confirming eligibility and completion of baseline data collection. Stratification was performed for opioid use (current versus past/never).
3.3. Outcome Measures
Outcome measures were taken at baseline, four, eight and 12 weeks, with the primary outcome being at four weeks. Evaluation occurred in the same manner and at the same intervals for all patients using standard strategies. Treatment failure was characterized as patients ending treatment for non-effectiveness or pursue of surgical or invasive procedures or other excluded co-interventions.
The primary outcome measure was the Oswestry disability index (ODI). The ODI is generally utilized as part of low back pain treatment trials, and gives a measure of functional change. The ODI was chosen with a subjective pain rating (23-25). Secondary outcome measure was the VAS pain score (0 - 10).
Data was collected at enrollment and subsequent treatments. Data after treatments termination was collected using the same tools for every participant by a blinded assessor.
3.4. Assessments
Timing of evaluations was identical for every patient. Evaluations were directed by blinded assessors. Enlistment and starting treatment occurred at the time of enlistment. All patients returned four weeks after initial treatment for evaluation of subject capacity to accurately perform the exercise, ask questions about the exercise, and to finish week four assessment. Subjective assessments were measured at baseline, and on the fourth, eighth, and twelfth week. Objective assessments were measured pre and post-treatment at baseline and post-treatment on the fourth, eighth, and twelfth week. No treatment was provided by the researchers. Patients all had the same and equivalent contact time with suppliers and assessors to minimize treatment differences. All patients were contacted on a weekly basis by a blinded assessor by phone to energize consistency and utilize day by day treatment diaries.
At every assessment, all patients received the same evaluation, including questionnaire and physical functioning assessment form. All measures evaluated utilization of computerized data collection methods at baseline and each follow-up.
3.5. Treatment Protocol
Every patient had identical quantities of visits and contact time. Every patient obtained uniform guidelines for the exercise. Patients were trained to perform the exercise every day after getting up and before sleep time, and up to two extra times during the day as required. Providers were trained in a standardized fashion to educate each participant.
There was no accepted standardized treatment found in the literature for chronic low back pain.
3.6. Compliance
Compliance was assessed by interviews and diaries. Total treatment time of 3.5 hours per week was set as the threshold for compliance. If VAS pain dropped to a daily average of less than or equal to two out of ten, patients were considered in compliance regardless of the number of treatments completed on that day. Patients were contacted once per week to promote compliance and answer questions.
3.7. Discontinuation
Subjects were encouraged to continue follow-up throughout the study. There were no penalties for early dropouts. Those who developed an adverse event were reported to the IRB.
3.8. Usual Care and Co-Interventions
Study participants were asked to avoid significant changes in medical/healthcare management of their LBP during the study period. All participants were allowed to pursue usual care with their own personal treating physician. No other treatment other than the Farshad exercise provided by the trial researchers. Usual care was limited to non-invasive therapies, including medications such as NSAIDS (prescription or over the counter) and opioids, physical therapy, manipulation, stretching and aerobic exercise. Self-applications of heat or ice were also acceptable. All usual care medications and interventions were recorded.
3.9. Interventions Not Allowed
Interventions that were not allowed included any lumbosacral invasive method, for example, discectomy, microdiscectomy, laminectomy, lumbar fusion, and disc desiccation by any chemical or thermal means. No infusions into the lumbosacral area other than localized trigger point without the utilization of glucocorticoids, and no utilization of oral or systemic glucocorticosteroids was allowed. Patients were given information about extra medicines at every assessment to determine continued eligibility.
4. Results
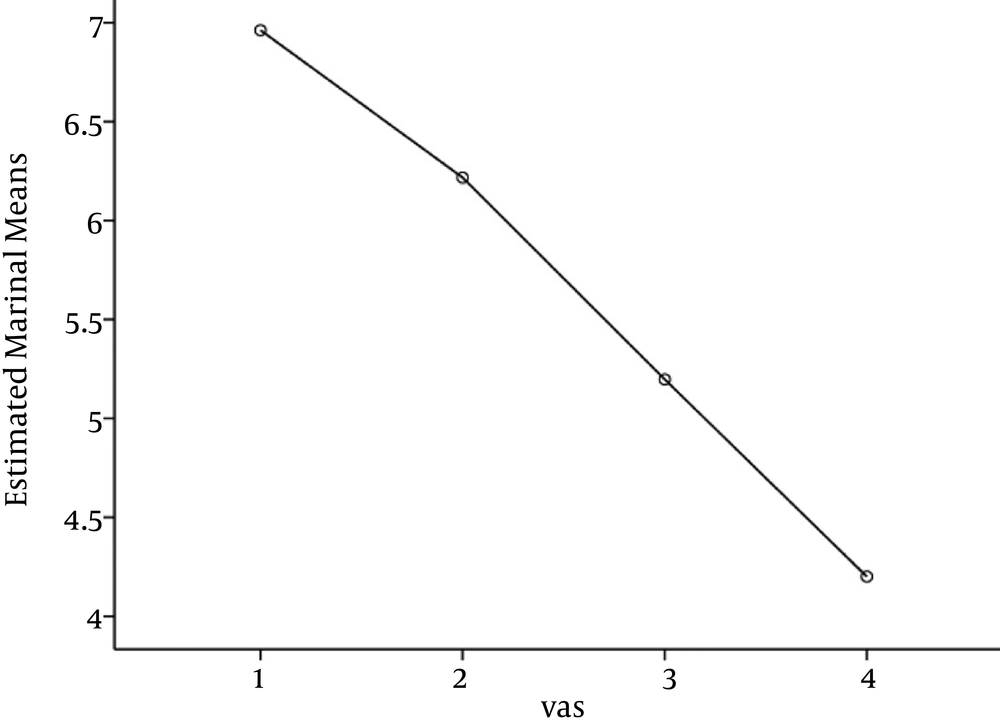
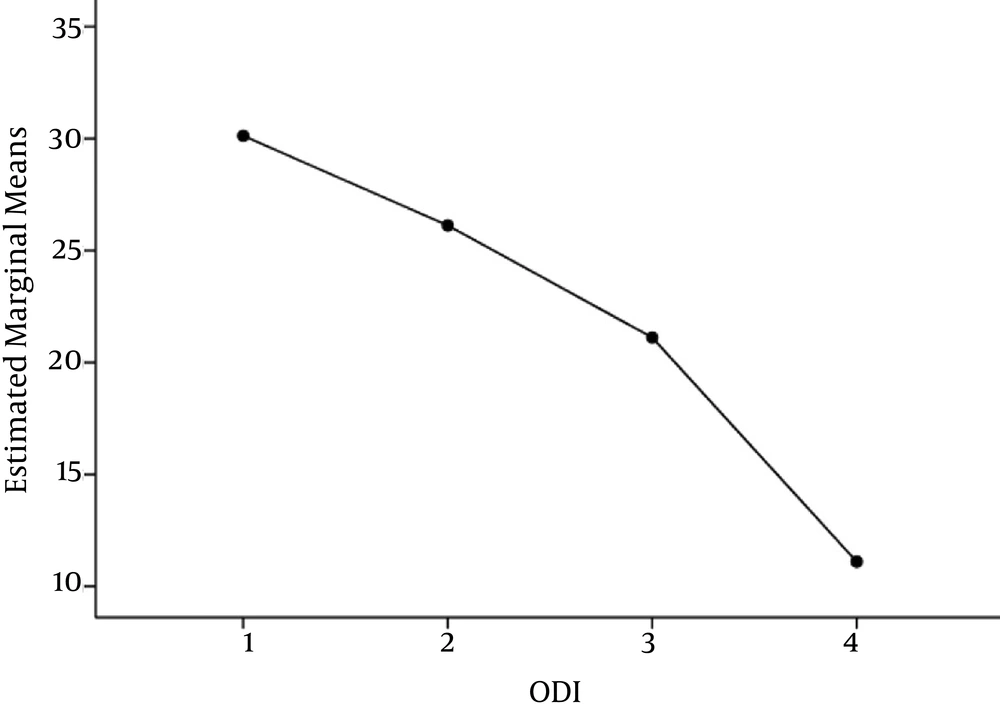
One hundred and thirteen individuals participated in the study including 47 females (41.6%) and 66 males (58.4%). The mean age was 41.7 (SD = 13.5) years old. Average pain scale at entrance was 7.0 (SD = 1.4) and ODI was averagely 30.1 (SD = 3.2).
Comparison of the pain scale in the four consecutive visits by repeated measured analysis of variance (ANOVA) with Greenhouse-Geisser test revealed a significant reduction of pain (P < 0.0001). Age and gender were not significant determinants of pain trend (P = 0.78, P = 0.64, respectively). Pairwise comparison between each visit with the next showed a significant improvement (P < 0.05).
Oswestry Disability Index was also reduced during the four sequential visits as compared with repeated measured ANOVA with Greenhouse-Geisser test (P < 0.0001). The most marked reduction was observed between the 3rd and 4th visits. Neither age nor gender had a significant impact on the response (P = 0.73, P = 0.69, respectively).
5. Discussion
Chronic low back pain is a critical issue, bringing about huge costs, lost productivity, and morbidity (1-6). Consequently, even little enhancements in treatment adequacy, especially for methods with few side effects, can meaningfully affect improvement of LBP. While there have been some studies showing the clinical adequacy of exercise, the effect is generally relatively small improvements in chronic LBP.
Farshad Exercise showed effectiveness in reducing disability and pain of chronic non specific low back pain patients.
All treatment questions will be directed to the interventionalists, as to maintain blinding of the assessors.
All treatment questions coordinated with the interventionalists, as to maintain blinding of the assessors.