1. Background
Head and neck cancer is one of the most common causes of cancer related death and oral cavity with 2% leading reason of death worldwide (1). Head and neck cancers are a heterogeneous groups of tumors originated from different anatomic sites. Prevalence of head and neck cancer related to diverse factors including age, race, sex and geography are differentially changed (2). Incidence of head and neck cancers is largely associated to smoking and alcohol drinking (3).
Free radicals as inevitably product in metabolic process in normal cells play important roles in cancer pathogenesis. Elevated reactive oxygen species (ROS) induces DNA damage, genomic instability and tumor suppressor genes reduction as well as increased expression of proto oncogenes (4). Increased levels of lipid peroxidation, shown by malondialdehyde (MDA) have been reported in different types of cancer. Conversely, the levels of total antioxidant capacity and related enzymes such as glutathione peroxidase, superoxide dismutase and catalase decrease in patients with head and neck cancers (5, 6).
Radiotherapy is the common approach in the treatment of head and neck cancers. Radiotherapy induces DNA damage in exposed cells producing high levels of ROS in site of tumor that ultimately cause cell death. Furthermore, radiotherapy changes signal transduction pathways leading to increased apoptosis rates in cancer cells (7, 8). Nonetheless, effects of radiotherapy on oxidative stress are minimally investigated with conflicting results. Some studies reported that levels of oxidative stress decreased following radiotherapy and conversely, enhanced levels of oxidative stress have been reported by some other investigations (9).
In the recent years, there has been an increasing interest in saliva-based analyses, because saliva collection methods are simple and noninvasive. Oral fluid sampling is safe for both the operator and patient and has easy and low-cost storage. Since saliva was put forth as a potential diagnostic tool, its use for surveillance of disease and general health has become a highly desirable goal in healthcare and medical research (10-13).
Increasing attempts to establish saliva as a diagnostic matrix have compelling reasons behind. In this regard, it clearly offers an inexpensive, noninvasive and easy-to-use screening method. In addition, it has several advantages over serum and urine for collection, storage, shipping and voluminous sampling. Moreover, handling of oral fluid during laboratory procedures is far easier than blood, because it does not clot, thus reducing the number of required manipulations. Furthermore, noninvasive nature of saliva collection approach could dramatically reduce anxiety and discomfort and thereby increase patients’ willingness to continue health-related examinations over time (14-16).
2. Objectives
Here, the objective of the present study was to evaluate oxidative stress status in patients with head and neck cancers before and after radiotherapy using measurements of lipid peroxidation and total antioxidant capacity levels.
3. Patients and Methods
3.1. Patients and Clinical Conditions
Whole intact saliva samples were obtained from 47 patients with head and neck cancers referred to Imam Khomeini Hospital (Tehran, Iran) for radiotherapy from January to September 2014, comprising 23 males and 24 females in pre- and post-radiation. Seventeen patients were excluded because of xerostomia and finally 9 males and 21 females evaluated in pre- and post-radiation. The mean age of patients was 46.8 years (ranged 17 - 62 years) and they were compared with a control group of 30 healthy individuals including 16 females and 14 males with a mean age of 27.9 years without any history of smoking and alcohol consumption. The conditions of all patients concerning antibiotic prophylaxis, nutrition and preoperative therapy were controlled. In this study, unstimulated saliva was used and saliva induction was prevented by avoiding patients and healthy controls for drinking, eating, smoking and all kinds of actions that may induce saliva secretions two hours before samples collection. Collected samples were kept at -70ºC until use.
Head and neck tumors were documented by two blinded expert pathologists. Patients with other diseases such as connective tissue pathologies, ulcerative colitis and vasculitis were excluded. The study was performed according to Declaration of Helsinki (2000) of the World Medical Association and all patients filled an informed written consent. Moreover, the study was approved by the Ethical Committee of Shahed University.
3.2. Determination of Lipid Peroxidation Using Malondialdehyde (MDA) and Total Antioxidant Capacity (TAC)
TAC of saliva was determined by measuring its ability to decolorization of ABTS radical cation according to previous fully described methods and the assay calibrated with Trolox (17).
Saliva MDA levels were determined by a method based on reaction with thiobarbituric acid (TBA) at 90 - 100°C (18). MDA and TBA react together in the TBA test reaction, to produce a pink pigment having an absorption maximum at 532 nm. The reaction was performed at pH 2 - 3 at 90°C for 15 minutes. The sample was mixed with two volumes of cold 10% (w ⁄ v) trichloroacetic acid to precipitate protein. The precipitate was palliated by centrifugation and an aliquot of supernatant was reacted with an equal volume of 0.67% (w ⁄ v) TBA in a boiling water bath for 10 min. After cooling, the absorbance was read at 532 nm. The results were expressed as pmol ⁄ mL according to a standard curve, which was prepared with a serial dilution of standard 1, 1, 3, 3-tetramethoxypropane.
3.3. Statistical Analysis
Differences of lipid peroxidation using Malondialdehyde (MDA) and total antioxidant capacity levels in saliva of patients and healthy control in various groups including pre-radiotherapy and post radiotherapy and comparison of these levels with control healthy group were analyzed by ANOVA and Tukey’s as Post hoc using SPSS software version 16.00 (SPSS Inc. Chicago, IL, USA).
4. Results
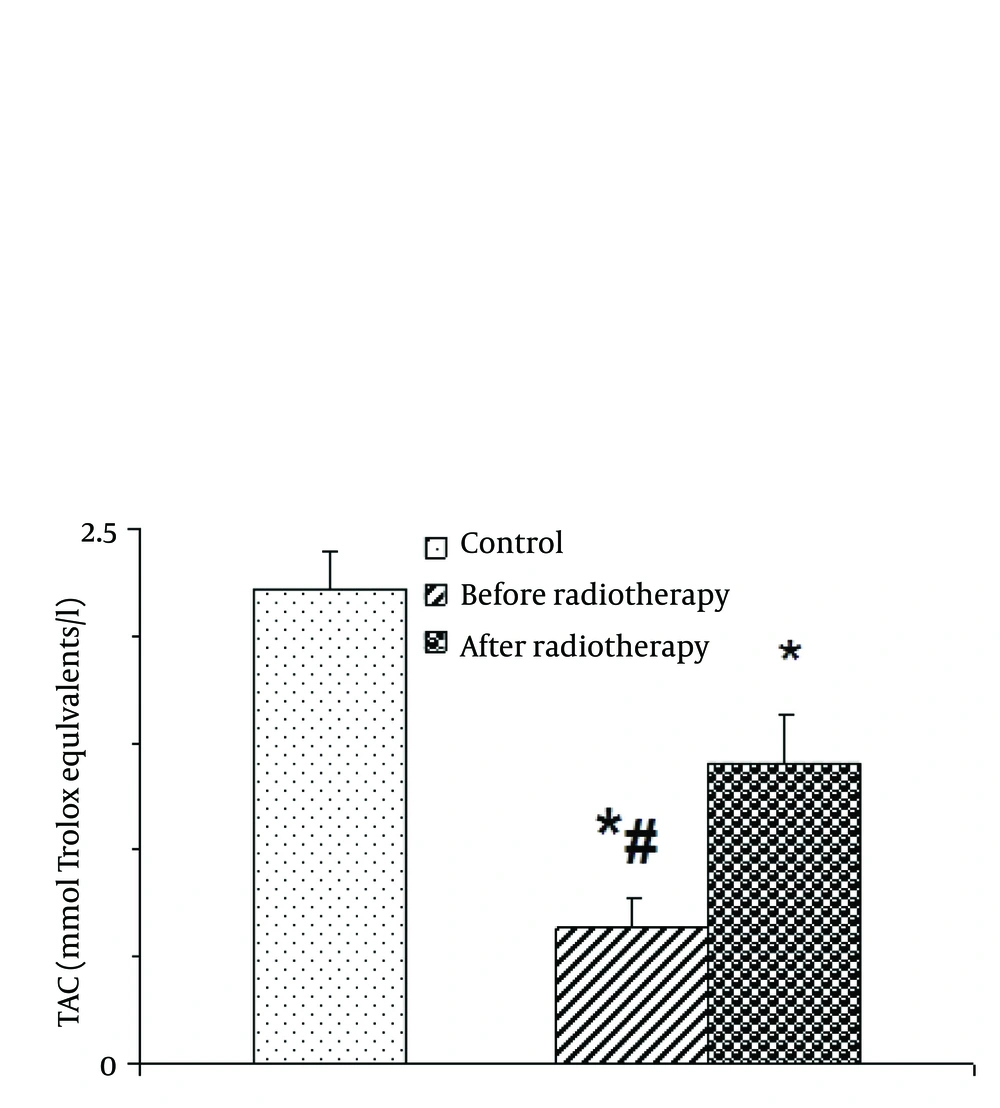
4.1. Levels of Total Antioxidant Capacity
One-way ANOVA indicated that saliva total antioxidant capacity was altered by radiotherapy [F (2, 87) = 20.635; P = 0.001] (Figure 1). Post-hoc analysis showed that TAC was significantly low in head and neck cancer group before radiotherapy than after radiotherapy and control group. Interestingly, the level of TAC was remarkably enhanced following radiotherapy in patients compared with before radiotherapy (P < 0.05).
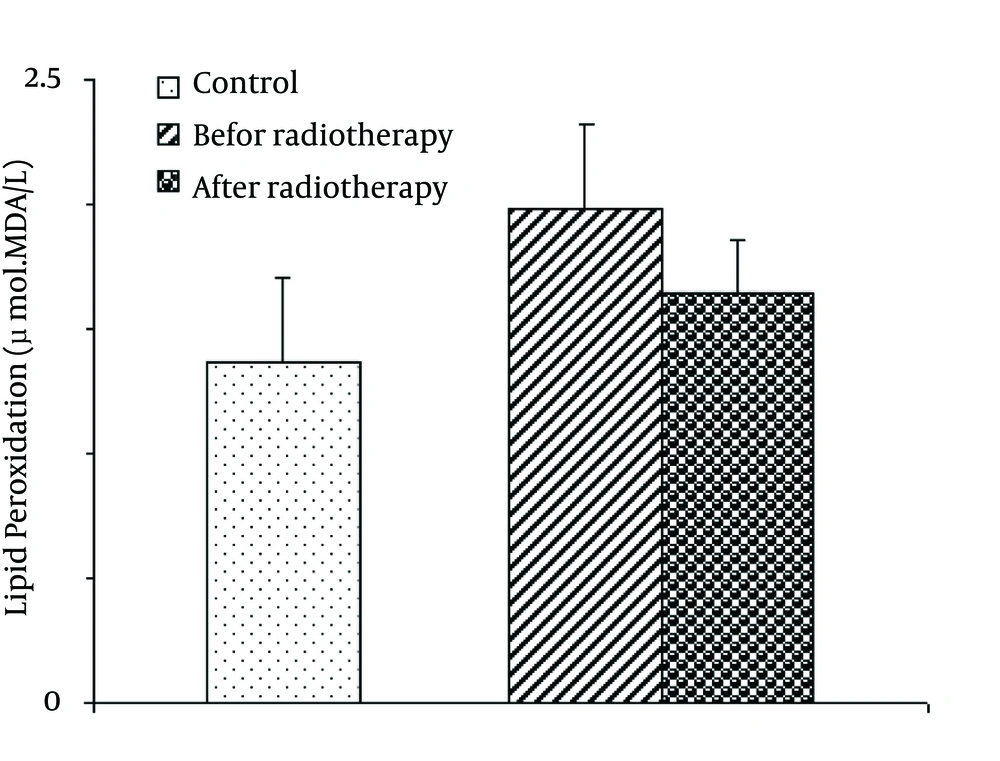
4.2. Levels of Lipid Peroxidation (MDA)
There was no significant difference in the lipid peroxidation as expressed by MDA levels among groups [F (2, 87) = 1.019; P = 0.365] (Figure 2).
5. Discussion
In the current study, total antioxidant capacity was low in patients with head and neck cancer before radiotherapy. After radiotherapy, the levels of total antioxidant capacity enhanced. Furthermore, lipid peroxidation was slightly high in patients with head and neck cancers, but the levels of MDA as marker of lipid peroxidation decreased following radiotherapy.
The oxidative stress induced lipid peroxidation to generate wide range of products including MDA. Previous investigations showed that MDA plays important roles in carcinogenesis through interaction with DNA and formation of DNA-MDA adduct. DNA-MDA adducts induce mutations in various genes such as tumor suppressor genes and oncogens as well as cell cycle alterations in several cancers (19).
Increased levels of lipid peroxidation alongside decreased levels of antioxidant capacity have been severally reported in patients with head and neck cancers (5, 20). Increased levels of lipid peroxidation and MDA are largely associated to damage red blood cell membranes enriched by polyunsaturated fatty acids. The levels of free radicals increase in head and neck cancers leading to enhanced lipid peroxidation. On the other hands, the levels of antioxidant capacity decreased following enhancement of ROS production in compensatory manner (21).
Free radicals, as mentioned above, play crucial roles in oxidative stress. Some studies showed that the levels of ROS products increased by radiotherapy resulting in enhancement of oxidative stress (22, 23). Furthermore, some authors indicated that radiotherapy-mediated oxidative stress decreased using antioxidant such as alpha tocopherol (24). Conversely, some other studies indicated that lipid peroxidation decreased after radiotherapy alongside improvement of antioxidant capacity status (21, 25). Decreased levels of MDA may be related to death of tumor cells and tumor load decline as the main source of ROS.
In conclusions, lipid peroxidation slightly reduced in patients with head and neck cancers after radiotherapy. However, antioxidant status seems to be improved in these patients after radiotherapy.