1. Background
Plantar fasciitis (PF) is one of the common reasons of heel pain, and manifest as pain originating from the insertion of plantar fascia near the medial calcaneal tubercle and is worse at the first step in the morning (1). Generally, PF is a self-limiting disease but the elimination time is often frustrating for patients. Several methods are known to relieve plantar heel pain, including changes in activities of daily living (ADLs), orthotics, stretching, taping and non-steroidal anti-inflammatory drugs (NSAIDs) therapy (2-4). The most favored method of nonsurgical treatment of PF is local corticosteroid injection. Few randomized controlled trials (RCT) debated the role of corticosteroids to treat PF (5-7). But it has potential complications and a high frequency of relapse and recurrence.
Todays, a degenerative pathology for PF is approved, rather than an inflammatory process. The histological evidence and chronic inflammatory changes with or without fibroblastic proliferation in the plantar fascia appear degenerative.
On the other side, the cytokines present in the platelet α-granules increase fibroblast migration and proliferation, vascularization and collagen deposition (8). On the basis of these findings, it was hypothesized that the treatment of PF with platelets rich plasma (PRP) should be more effective than corticosteroid injection.
The current study aimed to evaluate pain reduction with local PRP ultrasound-guided (US-guided) injections in patients with chronic PF and compare it with corticosteroid injection.
2. Objectives
The current study aimed to compare the effect of US-guided injection of PRP with corticosteroid injection to treat chronic PF cases. The level of pain for each patient was reported by visual analogue scale (VAS) and the degree of physical impairment and disability by Farsi version of the foot and ankle ability measure (FAAM).
3. Methods
3.1. Participants
Thirty patients with PF referred to the clinic that was included in the study. Inclusion criteria consisted of patients > 18 years, patients complaining of heel pain near the proximal plantar fascia on the medial calcaneal tuberosity with unilateral PF or pain prominent in one foot for a minimum three months duration and a VAS score of at least four at the medial calcaneal tubercle taking the first step in the morning; the subjects were randomly received either PRP or corticosteroid US-guided injection. Exclusion criteria consisted of using NSAIDs within 48 hours of procedure, corticosteroid injection at site of pain within the last month, having any other associated pathology involving the lower limbs, calcaneal fracture, calcaneal bone cyst, pregnancy, osteomyelitis, Achilles tendinopathy, abnormal erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) level, any systemic disorders such as rheumatoid arthritis (RA), haematological diseases and diabetes mellitus (DM).
For an alpha error of 0.05 and statistical power of 0.8, and according to other similar studies 15 subjects were required per group. After explaining the objectives and method of the study, the participants gave their informed consent. The patients were then randomized to receive either PRP or corticosteroid ultrasound-guided injection. Blinding of both participants and treatment providers was not possible in the study.
3.2. Study Treatment
With restricted randomization method (random allocation rule), 15 patients were treated by a local injection of 1 mL of methylprednisolone 40 mg and 1 mL of 2% lidocaine and 15 patients received injections of 2 mL PRP. The number of platelets in the baseline blood of this group of patients were measured before PRP preparation. The patients did not consume other drugs.
3.3. PRP Preparation and Application
A 17.5 mL blood sample with 2.5 mL of citrate, theophylline, adenosine and dipyridamole (CTAD) were drawn and collect in two sterile tubes. The tubes were then centrifuged at 1,700 revolutions per minute (rpm) for 12 minutes to separate the erythrocytes, the supernatant was first gently collected and then centrifuged again at 3,500 rpm for seven minutes. This method helped to achieve 3 mL of white blood cell containing platelets with rich plasma (4 - 6 folds increase over baseline platelet numbers). The number of platelets in the PRP was measured, and the concentration factor of the baseline blood platelets was calculated.
3.4. Injection Technique
The material was injected by a 5 mL syringe through a 25 gauge needle under guidance of US. The injection is best performed with the patient in the prone position with their feet hanging freely over the examining table and their ankles were 90° dorsiflexed. The beam is kept perpendicular to the plantar fascia to avoid anisotropy. The origin of plantar fascia was injected with a longitudinal view and medial approach. All patients were injected by the same person.
3.5. Post Injection Protocol
To utilize NSAIDs, any kind of foot orthoses or ice were prohibited. Since the patients might feel discomfort at the injection site for 48 hours, they were suggested to elevate their limb, modify activities and use acetaminophen for pain control.
3.6. Assessment Measures
The level of pain was evaluated for each patient by the VAS and the degree of physical impairment and disability was assessed by Farsi version of the FAAM (9). Additionally, by enrolling only the patients with unilateral PF or pain predominantly on one of their feet, they were clearly able to assess the relief from pain and disability by comparing it with the less painful side.
The VAS consists of a 10 cm horizontal line, that zero and ten reflect the total absence of symptoms and the worst imaginable pain, respectively. VAS assessments were recorded at baseline, three and eight weeks after treatment.
The FAAM includes two subscales: the activities of daily living (ADLs) and sports subscale. The ADLs and sports subscales contain twenty-one and eight questions, respectively, which estimate self-reported function and disability in the foot and ankle. The response to each of the ADLs and sport subscale items is scored from 4 to 0 (4 as no difficulty and zero as unable to do). Subjects completed the questionnaire at baseline, three and eight weeks after treatment.
Side effects of treatment were recorded after injection and at each visit (three weeks and eight weeks) by asking the patient about anticipated signs and symptoms and by physical examination.
3.7. Data Analysis
All data were analyzed using SPSS version 18.0. Kolmogorov-Smirnov test showed normal distribution of data. The VAS and FAAM subscales data were analyzed by 2 (drug: corticosteroid and PRP) × 3 (time: at the baseline, three and eight weeks after injection) repeated measures ANOVAs, and when the presented difference was significant, the Bonferroni post-hoc test was employed for multiple comparisons (P < 0.05).
4. Results
4.1. Characteristics of Participants
All 30 patients completed the study. The mean age was 44.1 years (ranged 27 - 63 years) with a mean pain history of 11 months (from three months to two years). There was no significant difference in baseline characteristics as well as baseline outcome measures between the two groups (Table 1). In the PRP group, the mean platelet concentration of PRP was 4.6 times compared with that of the whole blood.
| Patients | PRP (n = 15) | Corticosteroid (n = 15) | P Value |
|---|---|---|---|
| Male: Female | 7: 8 | 6: 9 | 0.713 |
| Mean age (years) | 44.7 | 43.6 | 0.625 |
| Mean pain duration (month) | 11.5 | 10.8 | 0.723 |
| Left side: right side | 8: 7 | 7: 8 | 0.715 |
| BMI | 28.2 | 30.3 | 0.683 |
| Baseline VAS score | 7.7 | 8 | 0.711 |
| Baseline FAAM ADL score | 64.3 | 57.7 | 0.691 |
| Baseline FAAM sport score | 48.3 | 48 | 0.695 |
Baseline Characteristics of Study Subjects in Each Group
4.2. Pain Relief
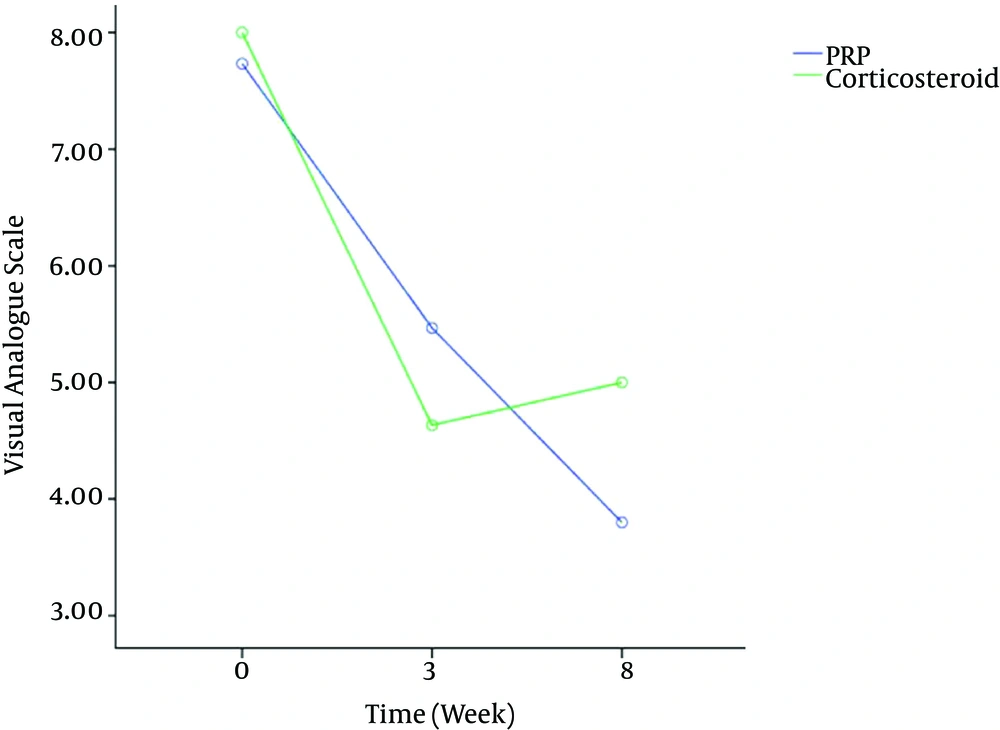
VAS data recorded at the baseline, 3three and eight weeks after PRP or corticosteroid injection are presented in Figure 1. This figure showed that, in both corticosteroid and PRP injected groups, the average VAS heel pain scores was statistically lower than that of pretreatment scores.
The 2 × 3 repeated measures ANOVA indicated no statistically significant drug × time interaction [df = 2, F = 1.87, P = 0.16] but a significant time [df = 2, F = 24.51, P < 0.001] main effect on VAS. Post-hoc analysis showed that VAS scores enhanced significantly from baseline to after three weeks (P < 0.001) and continued to eight weeks measurement (P < 0.001), but there was no significant VAS score improvement between three and eight weeks (P = 0.65).
4.3. Functional and Sport Improvement
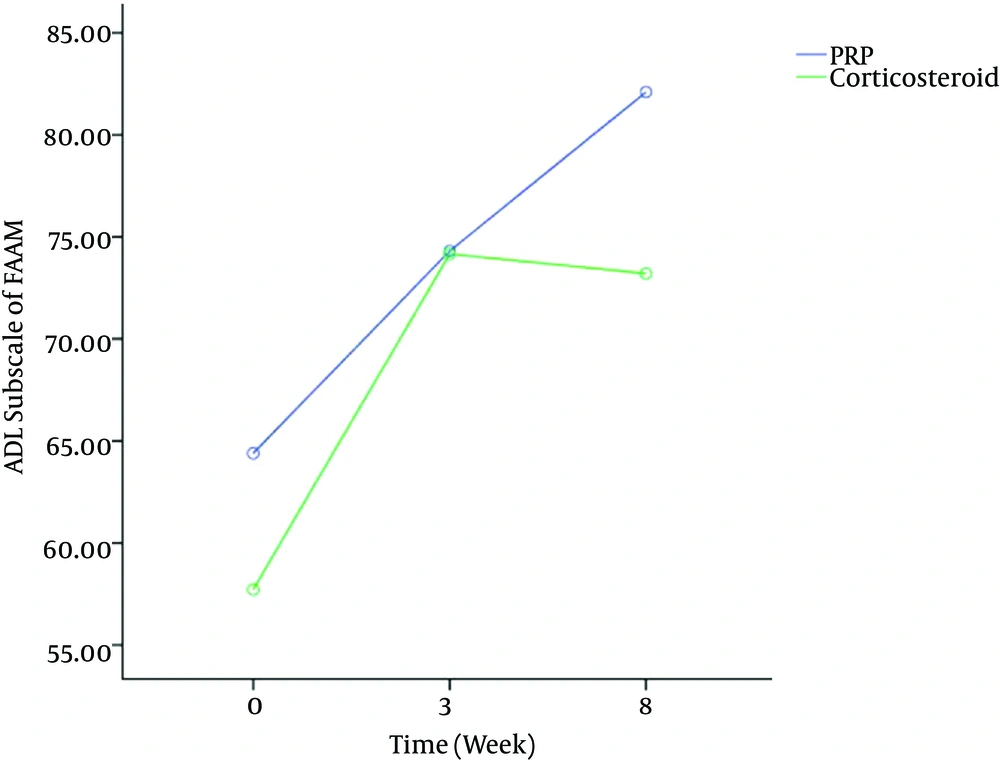
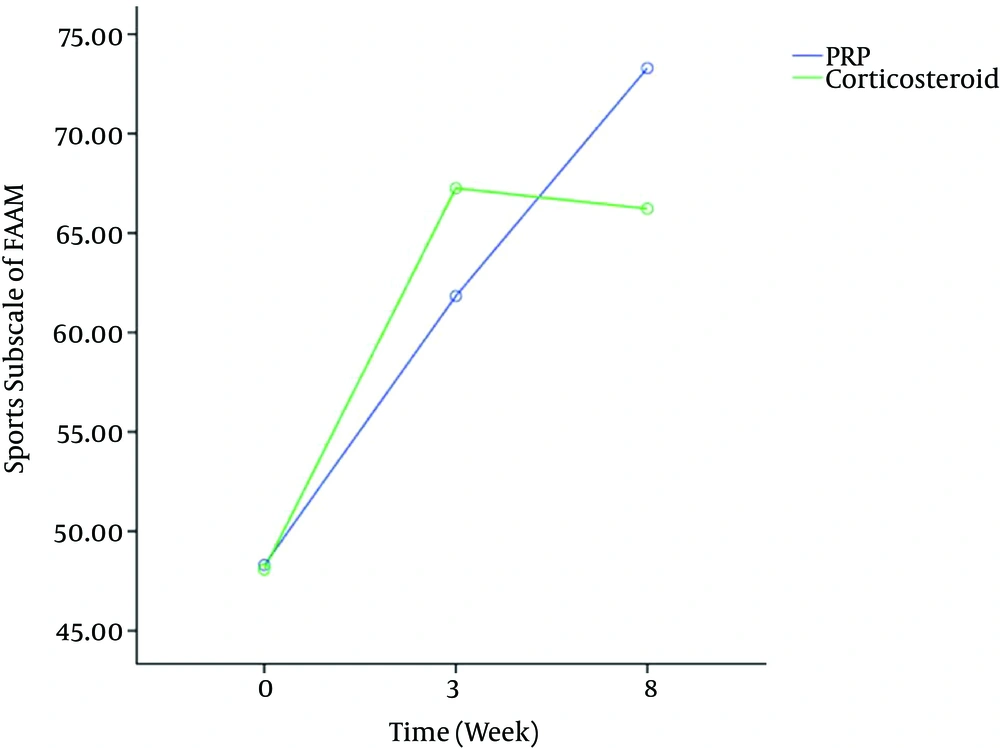
Figures 2 and 3 showed significant effect of time (P < 0.001) on FAAM scores. Bonferroni post-hoc test showed that FAAM scores significantly increased after three weeks (P < 0.001) and after eight weeks in both groups (P < 0.001), while the time × group interaction was not significant in FAAM ADL subscale scores [df = 2, F = 1.41, P = 0.25] and FAAM sport subscale scores [df = 2, F = 1.85, P = 0.16].
4.4. Safety Profile
None of the patients in either group experienced significant complications. Although five patients experienced severe pain at the site of injection (three in the case and two in the control groups), this generally subsided spontaneously within 48 hours.
5. Discussion
Recently, encouraging results are reported by PRP injection to treat muscle and tendon injuries and degeneration (10-13). The current study revealed that local injection of PRP furnishes consequential relief of pain and improvement in function that is comparable to the corticosteroid injection to treat PF. corticosteroid injection in PF, when conservative management is unsuccessful, is an effective treatment (14-16). But some authors concluded that corticosteroid injection can give short-term relief and seems to be useful only to a small degree apparently, since intrafascial injection may lead to permanent adverse changes within the fascial structure and since patients tend to overuse the foot after injection as a result of direct pain alleviation, fascial rupture is the side effect of repeated corticosteroids injections (17-19). Another important issue is thePF injection method. The current study used US-guided injection. There is evidence that US-guided plantar fascia injection can help with a reduction in plantar fasciathickness and pain; also there was no evidence of the rupture in plantar fascia at follow-up ultrasound examination; therefore, in some studies US-guided injection is suggested (14, 15, 20). While there are many studies in which PRP injection to treat chronic PF is beneficial, it is a controversial issue. Aksahin et al. (21) in their prospective, randomized controlled trial compared corticosteroid and PRP injections to treat PF. They reported that both methods impressively treated PF.
Shetty et al. (22) studied 60 patients and demonstrated the positive effect of PRP on PF after three months. This study described the comparison of an autologous platelet concentrate injection with corticosteroid injection in patients with unsuccessful non-operative treatment of PF. It exhibited that a single injection of autologous concentrated platelets decreased pain and improved function more than corticosteroid injection after three months. These improvements were sustained over time and complications were not reported. Ragab and Othman (23) reported a 60% success rate with PRP in patients with PF in three months follow-up. The same authors also documented a decrease in plantar fascia thickness, detected by ultrasound, over time when treated with PRP.
The current study observed highly significant differences between the groups regarding VAS and FAAM scores before and after treatment (P < 0.001) while comparisons of VAS and FAAM changes among control and PRP groups of patients showed insignificant differences (P > 0.05). It is noteworthy that in the current study the corticosteroid group was better at first (after three weeks) and then declined after eight weeks, but the differences were not significant; although the PRP group progressively improved. The current study results were consistent with those of Peerbooms et al. (24) who reported better response of corticosteroid group initially that declined later; in their study there was a significant difference in decrease of pain and disability of function following the platelet application after 26 weeks and one year for treatment of tennis elbow.
Another important issue is that there are different methodologies to prepare PRP. Various systems are available that permit preparation for outpatient use. To select a method, many factors should be considered, such as volume of blood drawn, rate of centrifugation, leukocyte concentration, type of anticoagulants, final PRP volume and platelet concentration. Due to differences in PRP attributes, reported evidence for clinical effectiveness of PRP cannot be generalized to all of these systems.
Controversies regarding the optimal quantity of platelets required for muscle and tendon healing and type of anticoagulants used for PRP preparation still persist. The current study used CTAD as an anticoagulant and achieved platelet count ≥ 4 ×, which seems to be effective due to previous studies (25, 26).
The current study had some limitation including the small number of patients and short period of follow-up; the small sample size made the study prone to error type 2 and short period of follow-up limited drawing final conclusions about the role of PRP injection to treat chronic PF. Future investigations should be conducted on a larger sample and with longer period of follow-up.
5.1. Conclusions
Local injection of PRP was a promising form of PF treatment. However sustained efficacy of this promising method and safer therapeutic options should be further evaluated in longitudinal follow-up studies that include a larger number of patients.