1. Background
Leishmania species are complex, flagellated, intracellular protozoan parasites. They infect human, primarily in the Middle East, Central Asia, and Africa and are prevalent in many other parts of the world including the tropical and subtropical regions (1-4). Leishmaniasis has a diverse group of clinical syndromes caused by the genus Leishmania (5). Zoonotic cutaneous leishmaniasis (ZCL), caused by Leishmania (L.) major, which is transferable from gerbils to human and has an increasing incidence rate, represents a serious public health problem (1). Gerbils are the main reservoirs for the parasite, and phlebotomine sand flies are the biologic haematophagus vectors (6).
The prevalence of infection is high in some provinces of Iran, such as Isfahan, Shiraz, Mashhad, Neishabour, Tehran, Khuzestan, Bam and Kerman. Isfahan is a well-known endemic area of ZCL. In the north east of Isfahan, the disease incidence is high, especially in rural areas (7, 8). Mirjaveh is an endemic region of cutaneous leishmaniasis (CL) and has become a new focus in border area of south-east of Iran, where L. major is the causative parasite species in this area. Frequent reports of CL have been recorded from the rural area of Chabahar (9). Chemotherapy is able to reduce the disease but does not consistently eliminate the infectivity of reservoirs and vectors hosts (10). This feature has promoted the development of vaccine against cutaneous leishmaniasis as an important tool and effective strategy for controlling cutaneous leishmaniasis caused by L. major (1). Successful immunization could significantly reduce the incidence of human cutaneous leishmaniasis.
Literature search for leishmaniasis vaccines has indicated the preparation of amastigote-derived antigens (11). The best novel protective antigen was an amastin-like gene (12). One of the antigens from amastigote stage is p4 antigen (13). Earlier studies have identified 35 kDa p4 of L. amazonensis and L. pifanoi which has the ability to stimulate Th1-type cellular responses in BALB/C mice challenged by L. Pifanoi promastigotes (3, 11, 14-16). Immunodepletion studies of p4-vaccinated mice indicate that CD4+ and not CD8+ T cells are critical for protection against L. pifanoi (17). P4 retained inside the endoplasmic reticulum and it is thought that p4 antigen has a role in RNA stability (gene expression) or DNA repair (3, 16).
2. Objectives
For the first time in Iran, to investigate host immune responses, to Leishmania amastigote-stage-antigens, we have under taken p4 gene expression.
3. Materials and Methods
This study was conducted in the cellular and molecular biology research center, Shahid Beheshti University of Medical Sciences of Tehran, Iran, in 2005- 2008. In a previous study, we had subcloned the L. major (MRHO/IR/75/ER) p4 gene into the pQE-30 expression vector (16).
3.1. Gene Expression
rpQE-30 was transformed into E. coli M15 strain. A bacterial colony containing rPQE -30 was inoculated into 5 mL X medium [1.2% Bactotryptone, 2.4% yeast extract, 0.04% glycerol, 1% M9 salts (6.4% Na2HPO4-7H2O, 1.5% KH2PO4, 0.025% NaCl, 0.05% NH4cl)] containing 50 µg/mL Ampicillin and was incubated overnight at 37°C and 200 rpm. The next day, the bacteria were subcultured into a 50-mL flask and placed in a 37°C shaker incubator at 200 rpm. Cultures in logarithmic phase (at OD600 of # 0.5-0.6) were induced for 5 hours with 1 mM Iso-propyl-ß-D-thiogalactopyranoside (IPTG) (18).
Samples were collected before and after induction and lysed in a 2X sample buffer (100 mMTris-HCL PH8, 20% glycerol, 4% SDS, 2% 2-ME 2%, bromo phenol blue) and separated on a 10% SDS-PAGE (19, 20). The gel was stained with Coomassie brilliant blue R-250. Bacterial alone and with intact plasmid (pQE-30) were analyzed in parallel.
3.2. Western Blot Analysis Using His-tag Monoclonal Ab
The proteins were electrophoresed on a 10% SDS-PAGE and transferred to a nitrocellulose membrane (20). Protein bands were fixed on nitrocellulose membrane using UV cross-linker, the membrane was blocked with 3% bovin serum albumin (BSA) at room temperature for 1 hour and washed twice with 1X TBS (10 Mm Tris, 150 mM NaCl).The nitrocellulose membrane was incubated with the His-tag monoclonal antibody as a primary antibody at a 1:1000 dilution for 1h at 37°C. The membrane was washed three times with 1X TBS, containing 0.1% Tween 20 (TBST) and incubated with conjugated sheep anti-mouse IgG horse radish peroxidase (HRP) at a 1:500 dilution for 1 h at 37°C as a secondary antibody (3, 20). Binding was detected by colorimetry using diamino benzoic acid (DAB) and H2O2.
3.3. Western Blot Using Human Serum to Cutaneous Leishmaniasis
Western blot was carried out as described previously (3, 16). Briefly, the membrane was incubated using cutaneous leishmaniasis sera (L. major) (procured from Center for Research and Training in Skin Disease and Leprosy Tehran University of Medical Sciences) antibody as the primary antibody (1:200) and rabbit anti-human IgG conjugate as a secondary antibody (1:500) and detected by colorimetry with DAB and H2O2.
3.4. Double Diffusion
Double diffusion was carried out using cell pellets (bacteria containing recombinant plasmid) as an antigen against a serum sample of an infected mouse, on 1% agarose gel. The agarose gels were then stained with Coomassie brilliant blue, to visualize the antigen-antibody complex precipitation arcs. Bacteria alone and with intact plasmid were analyzed in parallel (3, 18).
4. Results
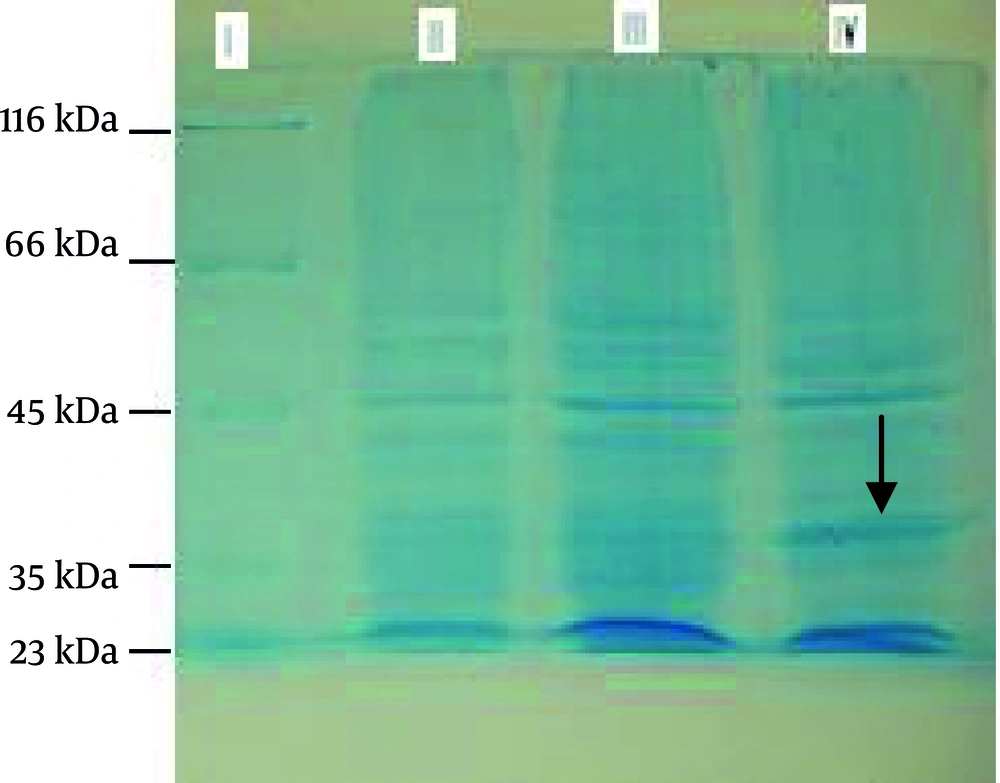
SDS-PAGE: rpQE- 30 was mass cultured and induced by IPTG. Samples were collected, lysed, and separated by 10% SDS-PAGE. Thus, rpQE-30 was expressed, and had an approximately 35-kDa molecular weight, after induction. Bacteria alone and with intact plasmid pQE-30 were analyzed simultaneously. Figure 1 shows the 10% SDS-PAGE of the lysate cells of bacteria alone, with intact plasmid and expressed recombinant plasmid (p4 protein).
Western blot: Cell lysate was electrophoresed on 10% SDS-PAGE and transferred to a nitrocellulose membrane. Western blot was carried out using two different antibody groups. Also, bacteria lysate alone and with intact plasmid were analyzed in parallel. Recombinant p4 protein were reacted to antibodies and not reacted by bacteria lysate alone and also intact plasmid (Figure 2, Figure 3). Figure 2 shows the western blot using His-tag monoclonal antibody as the primary antibody and sheep anti-mouse IgG horseradish peroxidase (HRP) conjugate as the secondary antibody.
Figure 3 shows western blot using human cutaneous Leishmania serum ( L. major ) antibodies as the theprimary antibody and rabbit anti-human IgG HRP conjugate as the secondary antibody.
Double diffusion: The pellet of bacteria containing recombinant plasmid (Ag) reacted with infected mouse serum (Ab). Figure 4 shows the antigen-antibody precipitation reaction. Bacteria alone and with intact plasmid were analyzed in parallel, but did not react with Ab as shown in Figure 4.
1: Bacteria lysate with the recombinant plasmid (rpQE-30), 5 h after induction, 2: Bacteria lysate with the recombinant plasmid (rpQE-30), overnight after induction, 3: Bacteria lysate with the recombinant plasmid (pQE-30-4), prior to induction, 4: Bacteria lysate alone 5: Bacteria lysate with the intact plasmid (pQE-30)
5. Discussion
Because of the wide spread of reservoir and vector hosts of L. major in nature, the treatment alone cannot control ZCL, therefore the development of vaccine can be an effective strategy. In an attempt to achieve an effective vaccine, using surface antigens of the parasites [(especially amastigote form (1, 12)] is an important tool, one of which is the p4 antigen (13).
Purified antigen p4 provided partial to complete protection in BALB/C mice against infection with L. amazonensis and have also been found to elicit a preferential Th1-like response in patients with American cutaneous leishmaniasis (15). Campbell et al. worked with L. amazonensis gene encoding p4 nuclease. Susceptible BALB/C mice were immunized with P4/HSP70 and it was reported that P4/HSP70 could be suitable in DNA-based vaccines for protection against new and old world Leishmaniasis (14). Farajnia et al. cloned the Class 1 nuclease from amastigote-stage of L. major (LmaCIN) which was a L. major homologue to the p4 nuclease (21) and it’s production elicited the immunological responses to immunoprophylaxy of leishmaniasis (22). As the first step toward evaluating this Ag as a vaccine, we expressed the Iranian L. major p4. The results of SDS-PAGE, Western blot, using His-tag monoclonal Ab and cutaneous leishmaniasis sera, (L. major), and double diffusion tests confirmed the success of p4 protein expression. We used pQE-30 plasmid as an expression vector, which has high quantity of expressed p4 protein (3, 23). Positive results from western blotting using cutaneous leishmaniasis sera is one of the first reports, this result, and the result from the double diffusion test proposed the hypothesis about using this Ag in diagnostic tests (3, 13).
In conclusion, the L. major p4 gene was successfully expressed and can now be used to continue the researches on vaccine production.