1. Introduction
Although infection with Fasciola hepatica and F. gigantica is found among cattle, sheep, goats, buffalo and some other ruminants such as horses and rabbits in most parts of the world, with rates as high as 90% were found among livestock in many countries (1), human infection was found with low incidence in Africa, Asia and South America (2).
Human fascioliasis has been increased during the last four decades and it has been estimated that 2,400,000 of human cases are found in 61 countries with 180 million inhabitants at risk (3). For example, in Egypt, where 27.7 million people estimated to be at risk and the prevalence rate is between 7 and 17% in its rural areas, the infection becomes an emerging health problem (4).
Reported in 1998 from 51 countries over 25 years, the total number of human cases was 7071. Of those, 3267 cases were from America, 2951 from Europe, 487 from Africa, 354 from Asia and 12 from Oceania. The increasing prevalence of human fascioliasis observed in parts of the world since 1980, has changed the status of the infection from secondary zoonotic disease to an important human parasitic disease and the infection is now considered as emerging or re-emerging infection (5).
The total number of people infected with fascioliasis reported from different countries until 2002 were estimated to be 830,000 in Egypt, 742,000 in Peru, 360,000 in Bolivia, 20,000 in Ecuador, and 37,000 in Yemen. In some countries such as Bolivia, prevalence was reported to be as high as 70% (6). In this country and in Peru, human and animal fascioliasis are considered to be hyperendemic (7).
In Peru, the number of human fascioliasis has been increasing in the last decades and become an important parasitic disease (8). In hyperendemic areas of Altiplano in Peru, located at a very high altitude of 3910 meters, the overall mean prevalence of Fasciola hepatica was 24.3% with a range between 18.8 and 31.3%, with up to 2496 eggs per gram of feces (9). Another place in which the outbreak of infection occurred was Eastern Anatolia in Turkey in 2008 (10).
Studies undertaken in high endemic countries during recent epidemic have shown marked different epidemiological situations and transmission patterns in endemic areas (11). High rate of human infection in epidemic forms were recently reported in some countries including Iran, where two epidemics of fascioliasis occurred between 1989-1999.
In Iran located in the Middle East, (Figure 1), infection of cattle, sheep, buffalo and goats with Fasciola hepatica and F.gigantica were reported from many parts of the country (12-14). For examples in one study, it was shown that in parts of Gilan, where human epidemy occurred, 71.4% of cows were infected with F. gigantica and 28.5% with F. hepatica . The average rate of infection with Fasciola among 445 cows in 3 areas of Gilan was 32.1% with rate as high as 55.2% (15). High rate of animal infection was also reported from other parts of Mazandaran Province. Coprological studies showed that 25.4% of cattle and 7.3% of sheep were infected with fascioliasis (16).
Our studies in 2003 showed that in Mazandaran Province , 6.72% of cows, 1.98% of sheep, and 0.87% of goats were infected. From two buffalos examined, one was infected. The average number of worms recovered from each liver of sheep was: 4.6% F. hepatica, 0.22 % F. gigantica and 0.6% Fasciola which their species could not be determined. The average numbers of each species found in liver of cows were, 1, 3.01 and 0.94, respectively (17). These results may indicate the higher role of F. gigantica in the transmission of fascioliasis among human (18). Similar results were reported by Ashrafi et al. in 2004 (19).
In addition to F. hepatica and F. gigantica, another species called F. indica found infecting cattle in Iran (14). Human infection with this species is reported from India and Korea (20). Some believe that this species is the same as the hybrid of F. hepatica and F. gigantica reported above (21).
Studies undertaken in some countries showed high financial losses due to infection of livestock with fascioliasis. For example in Switzerland, the financial losses due to bovine fascioliasis was estimated to be 52 million local currencies per annum which represents a median loss of 299 per infected animal. The losses arise from reduced milk yield and reduced fertility, meat production and the condemnation of livers (22). The animal fascioliasis especially infected with F. hepatica is a leading cause of production losses in cattle and sheep and other livestock , meat industries , reduced weight gain , milk production, and extinction. These economic losses due to infection of livestock with fascioliasis were well described in parts of Africa (23). In Iran, the economic loss due to Fasciola infection among livestock in a small slaughter house in Mazandaran found to be significant (16).
Regarding human infection with fascioliasis in Iran, sporadic cases were reported from many parts of the country until 1988 when the first outbreak occurred in Gilan (16, 24-27).
Before 1988, up to 100 cases of human infection have been diagnosed per year in Iran, sporadic cases in epidemiologic studies or from ectopic parasite in various organs are mentioned in old literatures. Reports in 1969 indicate an outbreak of eosinophilia with unknown origin, which possibly was caused by fascioliasis in Gilan (28). Presence of leptosporosis in Gilan which causes high eosinophilia should be considered in diagnosis of fascioliasis.
The first outbreak of fascioliasis, the largest in the world, occurred in Gilan in 1988. It began in February 1988. Total number of infected inhabitants estimated to be between 9008 and 20,000, but more accurate number might be 10,000, which is calculated based on the total population of infested city of Port of Anzali on the Caspian Sea which was 100,000. Although infection was found in all age groups, the highest rate was found in age groups below 35 years old. The peak of transmission was from February to June. In this outbreak, cases were diagnosed parasitologically and serologically in Gilan and Mazandaran, where 6 million inhabitants were considered at risk. Serological examination of 452 inhabitants of Anzali using ELISA method in 1988 showed infection rate of 50%. The rate was 34.95 as immunoelectrophoresis was used. Higher infection rates were found in Port of Anzali and the town of Taleghani. Among positive cases, 13.7% showed IgG, 26.5% had IgG and IgM and 37.6% showed IgM (29).
In one study, examination of 225 inhabitants in 9 districts of the Port of Anzali revealed a rate of 20% fascioliasis (26) and in another study, 45 were found infected from 458 persons examined (27). In Gilan, blood eosinophilia of 37.4% was found from 884 inhabitants, of whom 423 had Fasciola eggs in their stool (30). The outbreak lasted for 18 months, but examination of 2200 inhabitants from 44 villages in Lahijan did not show any infected cases after two years (31).
The number of infected cases found between 1984 and 1996 in Gilan was 1100, of whom 904 were in the Port of Anzali, 94 cases in Lahijan, 57 cases in Rasht, 9 case in Astaneh, 8 cases in Langroud, 5 cases in Soume`eh Sara and one case in Roudsar (32).
Serological tests undertaken by Assmar et al. on 452 inhabitants of 8 regions of Anzali Port for Fasciola antibody, using ELISA one year after the first outbreak of Gilan, showed that infection in females was higher than males and the highest rate was among age groups of below 20 years old. The highest rate was in the town of Taleghani (29).
In another study undertaken a year after the outbreak in Gilan, clinical examinations were undertaken for 2364 inhabitants of the infested areas: stool examination of 884 among this group revealed the presence of eggs of Fasciola among 36% of them.
Among patients with eosinophilia of 30% or more, 75% had passed Fasciola eggs in their stool. Seventy percent of positive cases were female and the highest prevalence was found among age group of 20 to 30 years old. The manifestations observed in patients were weight loss, epigastric pain, perspiration, mussel ache, fever, anorexia, coughing and hepatosplenomegaly (33).
Second outbreak occurred in 1999 among the population of the ports of Anzali in the Caspian Sea. The number of infected cases in this epidemy was estimated to be 2465.
In Mazandaran, all 107 human cases were diagnosed between the 1999 and 2002. No differences were found regarding the infection rates among various genders and age groups. Lower human and animal infections were found in the eastern Mazandaran (16).
The number of infected cases reported from health centers in parts of Gilan from 1998 to 2004 is shown in Table 1. As it is shown in this table, the highest number of cases was reported in 1999 and the cases were found among inhabitants until 2004. Most of cases were from Anzali Port (34).
| Reported From | No. Per Year | ||||||
|---|---|---|---|---|---|---|---|
| 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | |
| Anzali City Health Center | 211 | 2465 | 1161 | 348 | 445 | 143 | 331 |
| Rasht City Health Center | _ | 691 | 114 | 95 | 91 | 32 | _ |
| Other Health Centers | _ | _ | _ | 59 | 47 | 3 | _ |
In addition to two above mentioned outbreaks in north of Iran, 17 cases of human fascioliasis were found in a district of Kermanshah in 2000. The youngest patient was four years old and the oldest was 49 years old. 53% of the patients were female and the rest were male. All patients were farmers and 82% of them had a history of watercress ingestion in a period of 1 to 2 months before the admission. The highest number of cases was found during the spring of 2001 (35).
While the use of Praziquantel for the treatment of cases in infested areas showed no effect, Bithionol usage showed a cure rate of 69% among patients (36). Further treatment efforts using Triclabendazole showed this drug to be more effective than Bithionol (37). Oral administration of 10 mg/kg of Triclabendazole for 1-3 days in fascioliasis infested areas of Iran resulted in high cure rate among infected cases (38).
In another study, patients from Gilan who were treated with Triclabendazole but remained positive by stool examination and serologically were treated with a dosage of 1.5 g/d of Metronidazole orally for three weeks. Evaluation of cure rates undertaken 3 and 12 months after the end of therapy showed that all were negative observing serology and egg in stool examination (39).
2. Discussion and Conclusion
The epidemiological picture of human fascioliasis has been changed in last decades. The number of cases reported from several geographical areas has increased significantly The increasing prevalence of human fascioliasis observed in parts of the world since 1980 has changed the status of the infection from a not-prevalent zoonotic disease to an important human parasitic disease.
It is difficult to pinpoint all factors responsible for raising the number of human fascioliasis in Gilan and the outbreaks but we will discuss some contributing factors for these outbreaks.
The reasons for these outbreaks were considered to be higher number of rainfalls, especially during summer, resulting in increasing the population of snail intermediate hosts, higher infection rate of snail hosts, higher contact of inhabitants with infested water, and the use of locally made spices added to the food products.
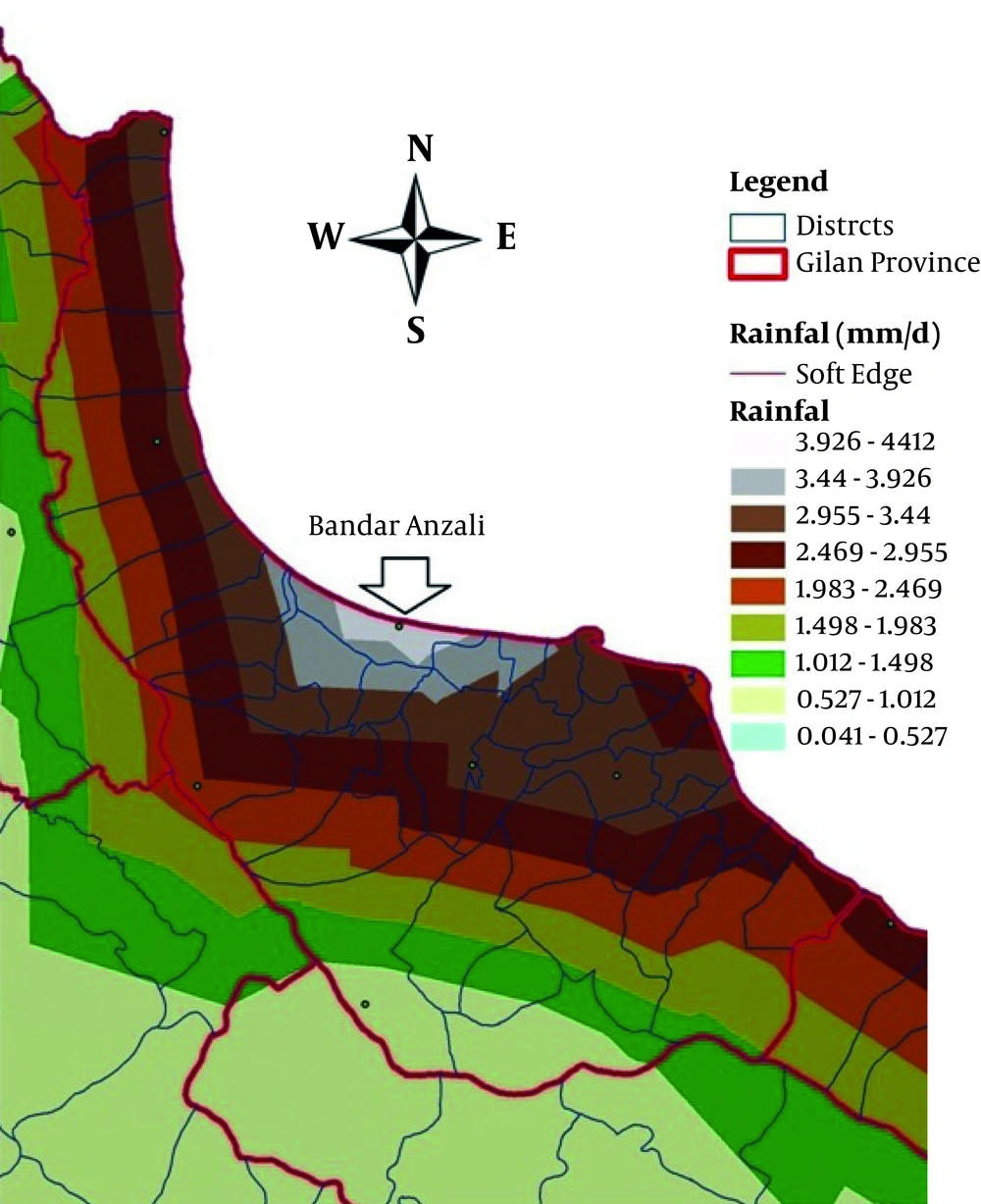
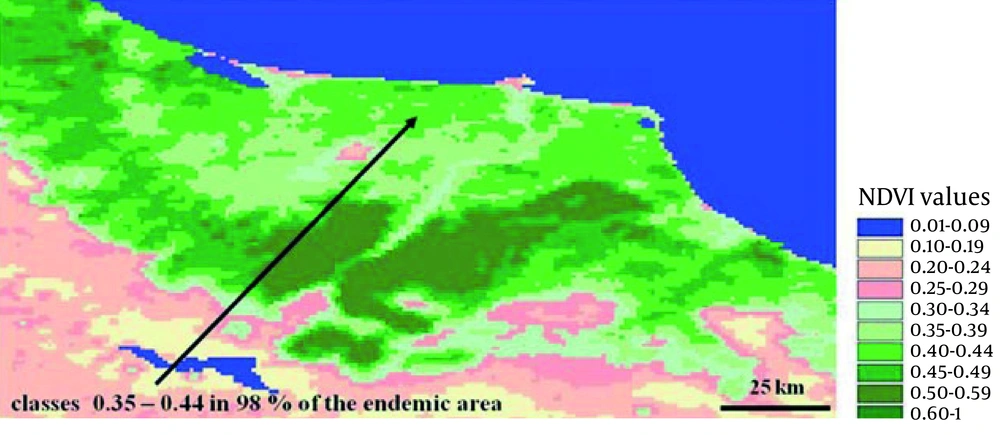
The amount of annual rainfalls in the port of Anzali was 1400 mm which is one of the heaviest rainfalls in the country. That heavy rain creates more suitable breeding places for the snail intermediate hosts. The relationship between summer rainfall and the higher incidence of fascioliasis was evident. The summer rain will reduce the salinity of the habitats of L. truncatula and thus higher breeding, density of snail. Figure 2 indicates heavy raining areas in Anzali and Figure 3 indicates above mentioned area with NDVI (normalized difference vegetation index) satellite images (40).
Heavy seasonal rainfall may also result in high rate of growth of wild plants and transmission of the infection. Our study using geographic information system (GIS) showed that higher number of infected cases is related to the higher normalized differences vegetation index or NDVI (40-42).
The main reasons of the outbreak were considered to be the consumption of product made locally from green aquatic aromatic wild grown plants of the Mentha and Eryngium species. These products called "Khalivash" and "Dallar" (so called Dallal, Dellal or Dellar in local accents) are added for spicing or as an appetizer in some locally made foods, which are eaten fresh (43). In a survey made in 1988, up to 91% of infected people had history of Khalvash consumption (28). Studies done in hyper endemic areas indicated that the ingestion of infective metacercariae, attached to these plants might be the main source of human fascioliasis (43-46).
Studies done in 2003 have shown that in areas where the mint planted by inhabitants is used for spicing the food, such as in the eastern part of Mazandaran, no case of infection was found, while in the western areas in Gilan where the wild mint is used, human fascioliasis is reported (45). Our study also showed that Mentha product is used less in Mazandaran and Gilan (28).
The possibility of having hybrid/interspecific cross-hybridization between F. hepatica and F. gigantica and the presence of an intermediate Fasciola species in Iran was considered by Bargues et al. (46), Periago et al. (47) and Moghaddam (16).
It is possible that F. gigantica and, possibly the intermediate form of Fasciola, are the main species of the Fasciola infecting man in the north of Iran (19). However, in most areas of Iran, including Gilan where outbreaks of human fascioliasis occurred, Fasciola hepatica and Fasciola species are simultaneously found in cattle and buffaloes. Results of a phenotypic study of adult flukes collected from bovines from Gilan using traditional microscopic measurements and an allometric model, show morphological differences between fasciolides of Gilan and standard species from geographical areas where both species do not co-exist (48).
Hybrid/interspecific cross-hybridization between F. hepatica and F. gigantica were found in other countries such as Korea (21) and Vietnam (49). Although F. hepatica and F. gigantica as well as intermediate forms cause human infection, the fluke species involved has not been determined in most areas (50). Studies using CIAS approach showed distinct morphologic differences between the intermediate species and the Fasciola hepatica and F. gigantica (16). Similar results were later reported (48) which used higher number of worm samples from naturally infected bovines in Gilan. The results showed that F. hepatica from Gilan is larger than the F. hepatica standard and Iranian F. gigantica-like specimens are longer and narrower than the F. gigantica standard, but with have smaller body (48).
Studies in Egypt have indicated the presence of F. hepatica, F. gigantica and intermediate forms and demonstrate the usefulness of computerized imagine analysis system (CIAS) for the phenotypic characterization of liver fluke adults from a fascioliasis endemic area (51).
Another method which might be more accurate for identification of F. hepatica and F. gigantica is the use of polymerase chain reaction (PCR) (52). F. hepatica and F. gigantica have similar lifecycles, but different distribution and transmission characteristics. F. hepatica is mostly found in high altitude and moderate climate, and F. gigantica is found in low altitude areas with subtropical or tropical areas (21, 53). It is believed that the main species of the Fasciola infecting man in the north of Iran is F. gigantica and possibly the intermediate form of Fasciola (19, 48).
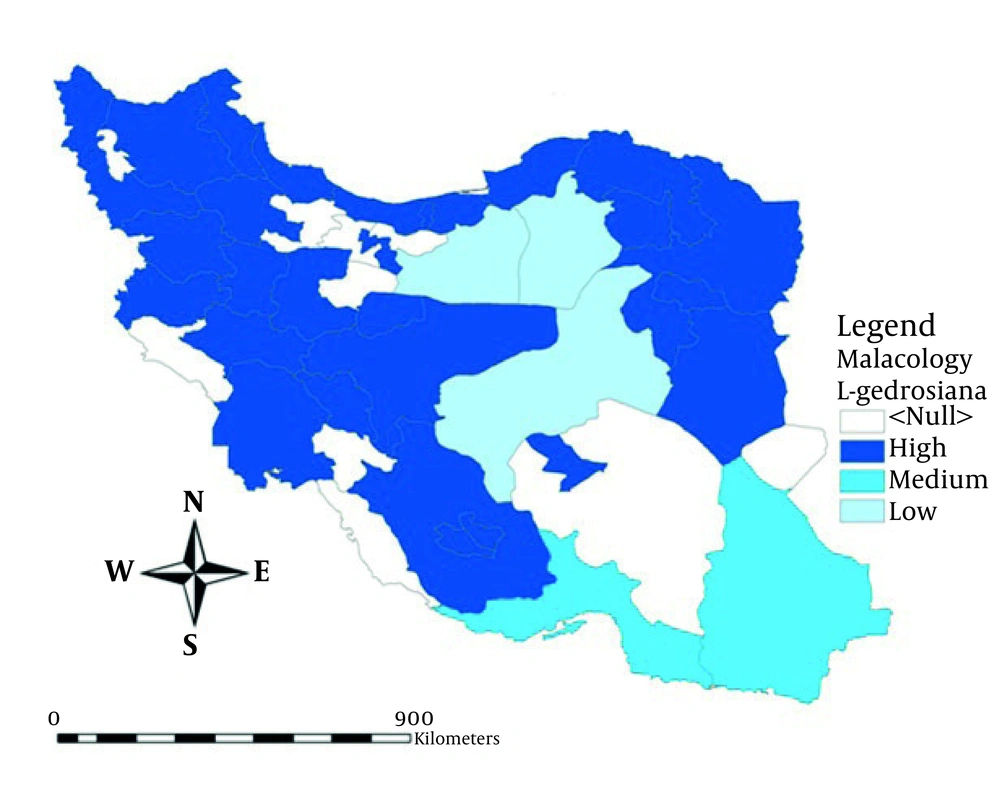
The snail intermediate hosts of fascioliasis in Iran proved to be Lymnaea truncatula and L. gedrosiana (54-55). Another Lymnaea species reported from north of Iran is Lymnaea peregra which its role in transmission of the infection is not known (54). Studies by Mansourian on vector snails in north of Iran indicate the presence of L. truncatula, L. gedrosiana and L. palustris (55).
Although according to some reports (56), L. palustris may play a role in transmission of fascioliasis, our studies did not show the role of this snail in transmission of the infection in north of Iran (34, 41, 57). The presence of L. truncatula in almost all parts of Iran, except in Busheher Province , and presence of L. gedrosiana in every parts of the country where malacological surveys were made (55, 58), indicate the potentially higher infection with fascioliasis in most parts of the country.
Our studies showed that the main breeding habitats of Lymnaea species in the north are the banks of rivers and rice fields, especially in the rice fields with permanent water which may constitute the larger and main breeding habitats of snail vectors (28, 41).
Our studies using SSUrDNA characterization of Lymnaea truncatula in the north of Iran have shown differences in the genomic pattern of snails (41). The similar result was found for Lymnaea species vectors in South and Central America (59).
The other reasons for the first outbreak is thought to be the entrance of the slaughter house sewage, containing the carcasses and remains of infected animals and eggs of the parasite into the snail habitats, also live infected livestock may cause snail vectors nfection when their excreta containing Fasciola eggs enter into habitats of the snail vectors. We have found higher infection among cows in western part of Mazandaran, where cows are wandering around without restriction, than in the east of this province where cows are restricted in their movement and have less contact with water (16, 28).
Because stool examination for Fasciola eggs is not a sensitive method for diagnosis of cases, immunodiagnosis method was tried. Results of studies in Iran showed that the standardized diagnostic ELISA for human fascioliasis based on the detection of IgG responses to parasite ES (excretory-secretory) is an accurate method for diagnosis of human fascioliasis (60, 61).
Although Triclabendazole has been used successfully to treat human cases of fascioliasis in Iran, resistance to this drug has been reported from a number of European countries (62). Although the possibility of higher endemicity and larger distribution of human fascioliasis and other water-born helminthiasis due to global warming was considered by some workers but not yet confirmed, the effect of globalization and higher number of travelers to other countries as tourists or immigrants may cause appearance of the infection in new areas (63). As floating cercaria may not be fixed by routine disinfectant agents, it is highly recommended for eco-tourists, foresters and other filed workers not to drink un-treated water, and prevent eating raw or semi-cooked wild herbs specially water-born or aquatic herbs in Iran.
Because of the importance of human fascioliasis, new strategy should be established for the better prevention, case finding and control of the infection. Figure 4 showed distribution of important intermediate hosts, G. truncatula and R. auricularia in Iran.