1. Background
Diabetes mellitus is an endocrine disease characterized by an insulin production deficiency or its resistance that results in an alteration of the metabolism and regulation of blood glucose level. It is categorized, according to its etiology, as type 1 or 2. Type 1 of diabetes mellitus results in the destruction of the beta cells of the pancreas, causing absolute deficiency of insulin, while type 2 results from cellular dysfunction in resistance to insulin by peripheral tissues (1).
Recently, there is an increasing interest in saliva-based analyses, because saliva collection methods are simple and noninvasive. Oral fluid sampling is safe for both the operator and the patient, and has an easy and low-cost storage (2). Since the saliva was put forth as a potential diagnostic tool, its use for surveillance of disease and general health, has become a highly desirable goal in healthcare and medical researches (3).
Increasing attempts to use saliva as a diagnostic matrix has compelling reasons on behind. In this regard, it clearly offers an inexpensive, noninvasive, and easy-to-use screening method. In addition, it has several advantages over serum and urine in terms of collection, storage, shipping, and voluminous sampling. Moreover, handling of oral fluid during laboratory procedures is far easier than blood because it does not clot, thus reduces the number of required manipulations. Furthermore, the noninvasive nature of saliva collection approach could dramatically reduce anxiety and discomfort, and thereby increases patients’ willingness to continue health-related examinations over times (3-7).
There is a controversy regarding the relationship between the concentration of blood and salivary glucose level in the literature. Several authors reported that an increase in the saliva glucose level of patients with diabetes (8-11); however, this relationship has not been confirmed in other studies (12-17).
2. Objectives
Since the number of patients with diabetes mellitus has been increased recently, an easier method of self-monitoring of blood glucose level is needed. To analyze the saliva as a diagnostic specimen in clinical practices for detection of diabetes mellitus, we compared salivary and serum glucose levels in patients with diabetes mellitus with healthy people. Here, we show that salivary glucose levels correlate with its serum concentrations.
3. Patients and Methods
3.1. Study Design and Population
The protocol was approved by the ethics committee of AJA University of Medical Sciences, Iran, and all subjects filled the informed consent forms before participation in the study. This study was designed as a case-control survey in Beasat Hospital of AJA University of Medical Sciences to investigate the correlation between serum and salivary glucose levels in patients with diabetes mellitus and healthy people. In this study, saliva and blood sample were obtained from 30 patients with diabetes and 30 individuals without diabetes. Patients who were hospitalized for side effects of diabetes mellitus with fasting blood sugar (FBS) level over 134 mg/dL were considered as case group. Age- and sex-matched healthy control subjects were selected from hospital staff or individuals who accompanied with patients referred to the hospital. People with lesion(s) in their mouths were excluded from the study.
3.2. Sample Collection
Fasting blood and saliva collections were carried out in the morning. For saliva sampling, all participants received detailed information about the collection protocols. Whole of the stimulated saliva was collected under resting conditions. Pre-stimulation was accomplished by chewing a piece of standard size paraffin and after 60 seconds, the participants were asked to swallow the saliva pooled in the mouth. Thereafter, whole stimulated saliva was collected for about 5 minutes into a dry, de-ionised and sterilized plastic tube.
Two mL of venous blood was drawn immediately after saliva sampling. Upon completing sample collection, the specimens were centrifuged at 3800 g for 10 minutes, and then the serum and saliva supernatants were isolated and stored at -70°C for later analysis of glucose.
3.3. Laboratory Measurements
Serum and salivary glucose levels were measured by an enzymatic colorimetric GOD-PAP assay, using commercial kits purchased from ZiestChem diagnostics company (Tehran, Iran). The measurements were performed by a spectrophotometer on the wavelength of 500 nm.
3.4. Statistics
Results are presented as mean SEM. Comparison of means for detecting the differences between groups was carried out with unpaired two-tailed student t-test. The Pearson correlation test was applied to determine the association between serum and salivary concentration of glucose. Results were considered statistically significant (P < 0.05). Analyses were performed using SPSS software version 16.
4. Results
As it was mentioned in methods, we closely matched the sex and age of control individuals with those of case subjects, so that the number of men and women, and also their age were nearly equal in both study groups. In each group, 20 men and 10 women aged of 25-71 years old (median: 56 years for patients and 55 years for healthy subjects) were recruited to the study. Body mass index (BMI) was significantly higher in patients than healthy individuals (P = 0.001) (Table 1).
| Clinical Characteristics | Healthy Individuals | Diabetes Mellitus |
|---|---|---|
| Age, Mean (SD), y | 52.7 (1.9) | 53.7 (1.2) |
| Sex (male/female) | 20/10 | 20/10 |
| Body mass index (BMI), Mean (SD), Kg/m2 | 22.1 (2.0) | 25.1 (3.3) |
From 30 patients with diabetes, two of them had type 1 and 29 of those have type 2 diabetes mellitus.
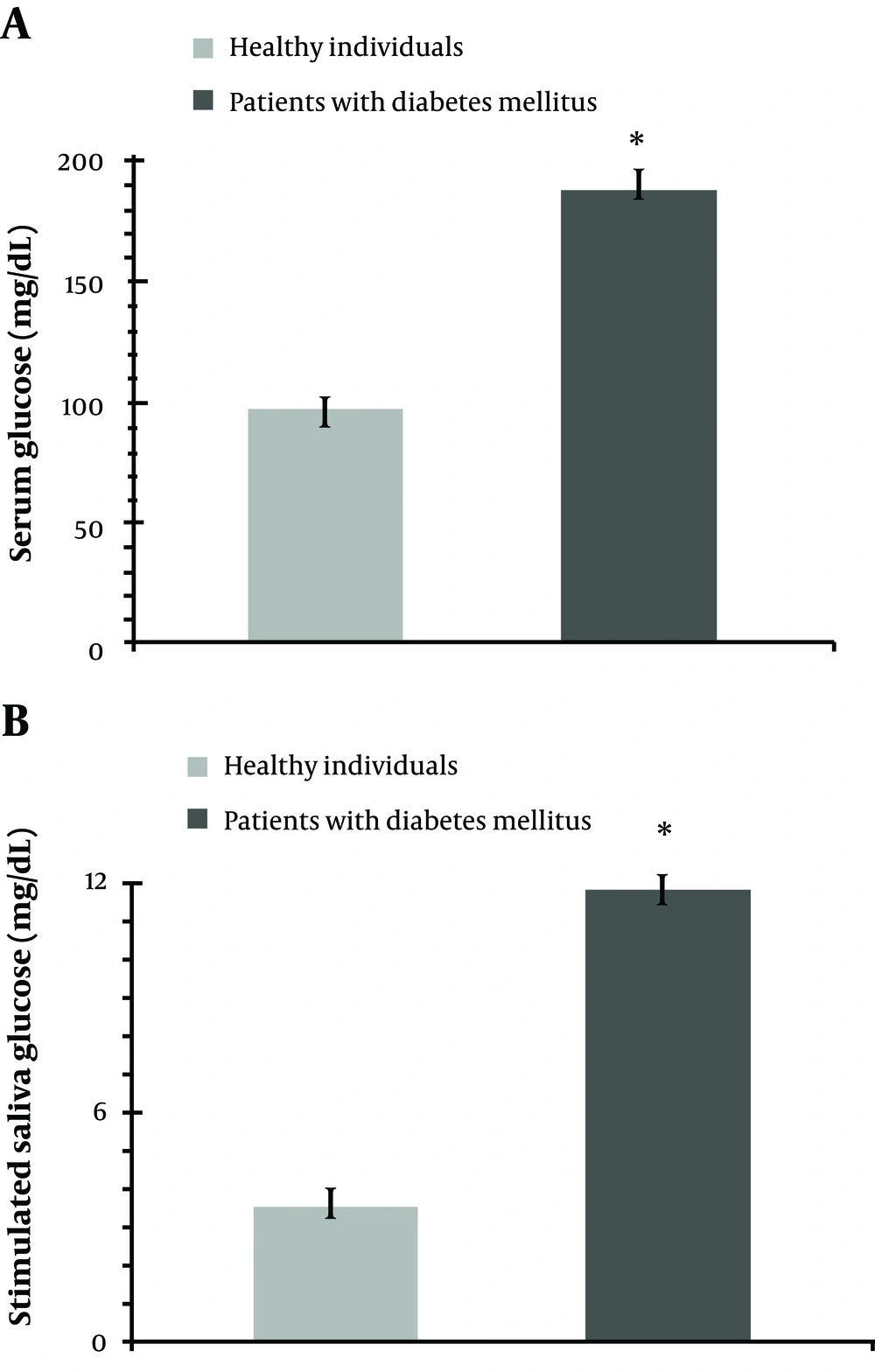
As expected, the mean of serum concentration of glucose was higher in patients with diabetes mellitus than that of the controls (Figure 1a). Serum glucose level ranged 134-336 mg/dL in patients with diabetes, and 74-118 mg/dL, in those without diabetes.
Stimulated salivary concentration of glucose proved to be significantly higher in patients with diabetes mellitus compared to control subjects (P = 0.001) (Figure 1b). Saliva glucose ranged 9-15 mg/dL in patients with diabetes and 0-7 mg/dL in control group.
Statistical evaluation of data using Pearson analysis indicated that serum glucose concentration correlates with salivary concentration of glucose (r = 0.64; P = 0.001)
5. Discussion
The number of diabetic mellitus patients has increased recently. In this study, the relationship between serum and saliva glucose levels in the patients with diabetes was investigated. We found that the stimulated salivary glucose concentration was higher in patients with diabetes than individuals without diabetes and it correlates well with serum glucose level which are in agreement with other studies (1,8,9,14,18). However, it differs from the results of other reports (1,13,14,18-20). It appears that the monitoring of glycemia in saliva of patients with diabetes is a viable alternative.
The rationale of our study for measuring glucose level in salivary secretions is that saliva is being considered as a diagnostic fluid of the future. Saliva is believed to be a mirror of the body, and may be acknowledged as a promising medium for monitoring health and disease states of an individual in healthcare programs. Several lines of evidence have consistently validated and proposed using salivary assays for diagnosing, monitoring, or predicting prognosis of diseases. In this regard, it has been shown that several biochemical molecules can be measured in oral fluids of patients, including, steroid hormones such as cortisol, (6) progesterone (7) and 17β-estradiol; (21) protein/polypeptide hormones such as creatine kinase MB, (22) creatine phosphokinase (23) and parathyroid hormone (24). Much of the attention to the saliva as a biological specimen is due to the quick, uncomplicated, and non-invasive nature of sample collection (5). Furthermore, oral fluid sampling is safe for both the operator and the patient, and has easy and low-cost storage. To establish saliva as an alternative medium of plasma for various biological assays, there must be a high correlation between plasma and saliva levels of measured parameters (22).
Conclusions: Based on the findings of this study, it can be concluded that salivary levels of glucose reflect the serum values.
Clinical significance: The core of the present study is the suggestion that salivary glucose can be used as an alternative of serum glucose for diagnosis and monitoring diabetes mellitus. Further studies are needed to be done to make this suggestion come true.