1. Background
Alcohol consumption, especially its binge consumption, is one of the most serious health hazards in the world (1, 2). The alcohol percentage content of beverages in Europe is 3% while it is 40% in our country (3). In any case, the problem of alcoholism is undeniable and unfortunately, alcohol consumption is a precondition for many social and physical harms. Cancer of the mouth, throat, larynx, esophagus, liver, and breast are all related to alcoholic beverages (3). Studies have shown that ethanol consumption can increase blood pressure, lipid peroxidation, protein oxidation, changes in lipid profile, and secretion of leukocytes into the coronary artery and cardiac muscle (4). In contrast to the destructive effects of alcohol, a complex antioxidant system containing enzymatic and non-enzymatic systems eliminates and neutralizes free radicals, and hence prevents the detrimental effects of these materials on cellular macromolecules. The antioxidant enzymatic defense system mainly consists of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) enzymes. The long-term consumption of ethanol not only increases the production of free radicals, but also reduces the activity levels of the antioxidant defense system, which leads to oxidative stress with destructive results (5). In this regard, the prevention of alcohol abuse requires the application of multiple theories in various scientific disciplines (3). Many reports refer to the therapeutic role of exercise in treating and even preventing many diseases. They consider sports as a valuable contributing factor to the treatment of addiction (6). In addition, in normal conditions, free radicals are the byproducts of oxygen metabolism in the body, which can degrade cell membranes, react with genetic materials, and cause the development and progression of many diseases (7). However, during intense endurance (aerobic) activities, the production of reactive oxygen species (ROS) increases and it is thought that the main source of these substances is mitochondria of active muscle cells (8). Several studies have been conducted on the effect of exercise on the antioxidant system, some of which showed that long-term exercise improves the antioxidant capacity in animal models (9) and humans (10). Moreover, exercise training reduced the oxidative stress associated with ethanol consumption and aging in three-month-old rats (11). In a study, the interaction of endurance training and ethanol consumption on rats’ heart tissue was evaluated and SOD, CAT, xanthine oxidase, and lipid peroxidation factors were evaluated. In the mentioned study, it was shown that with increasing age, antioxidant enzymes decreased and oxidative stress increased. In addition, alcohol consumption in both elderly and younger age groups decreased the antioxidant enzymes and increased oxidation. Besides, the training and alcohol consumption group showed an increase in antioxidants and reduced oxidants compared to those who consumed alcohol but did not exercise (12). On the other hand, antioxidant substances can be effective in the improvement of many diseases. One of these materials is curcumin, which is an effective ingredient of turmeric (diferuloylmethane) and has antioxidant, antibacterial, antifungal, antiviral, anti-inflammatory, and anti-proliferation properties. Curcumin inhibits the absorption of neutrophil granulocytes and myofibroblasts. It binds to thyroxin reductase (TR) and converts it into nicotine amide adenine dinucleotide phosphate (NADPH) oxidase, which prevents the formation of ROS (13). Many studies have reported the protective effects of curcumin against various diseases (14-16). Considering the literature, no study has been conducted to examine the antioxidant effects of exercise training and curcumin consumption on the heart in the course of alcohol consumption or its withdrawal.
2. Objectives
Accordingly, concerning the harmful effects of extreme alcohol consumption, this study aimed to compare the effect of swimming training along with curcumin consumption on SOD and GPX protein levels in rats’ heart tissue after the withdrawal of binge alcohol consumption.
3. Methods
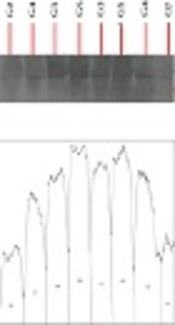
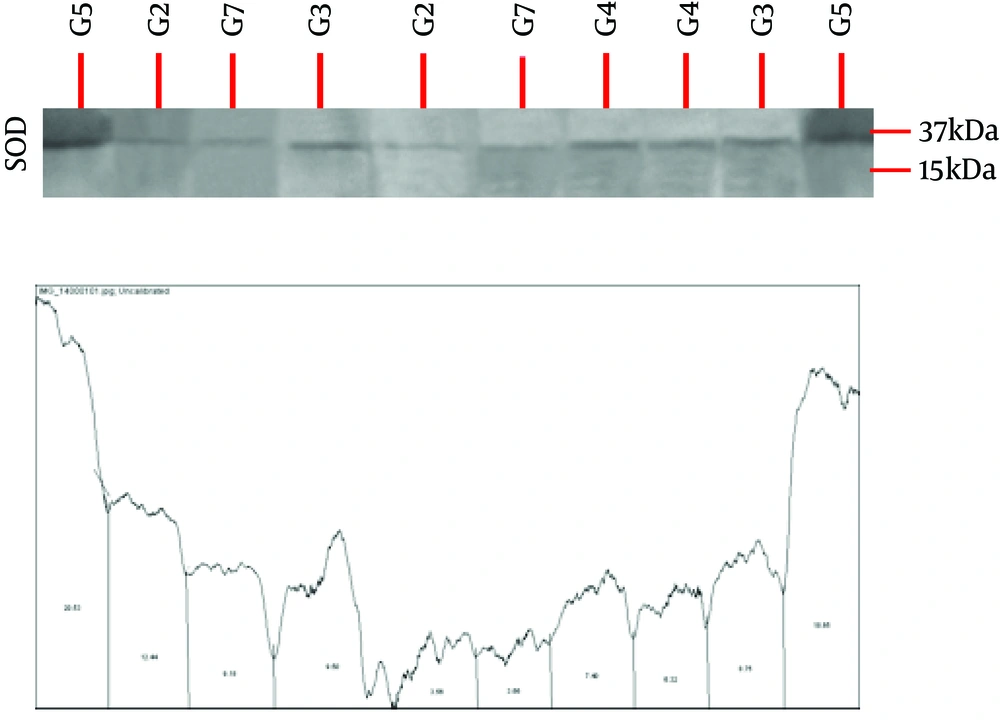
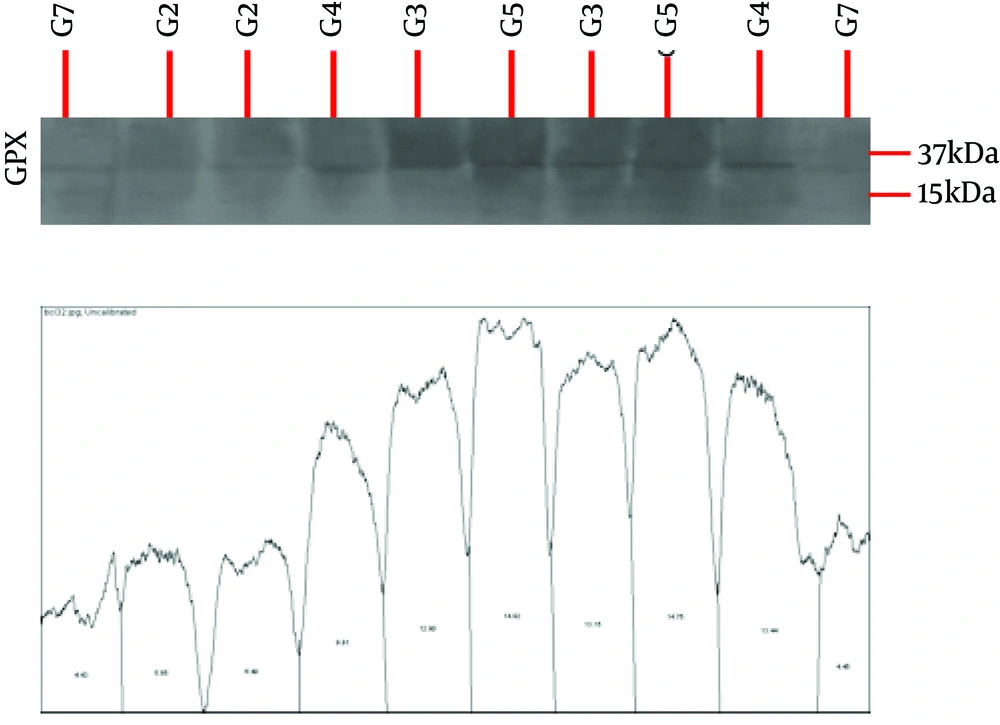
In this experimental study, 40 male Wistar rats (200 ± 50 g) were purchased from the Animal Breeding Center of Pharmacology Department at Tehran University of Medical Sciences and maintained in the animal’s house of this university in order to adapt to the conditions. Standard environmental conditions were kept in terms of temperature, humidity, nutrition, and 12-hour-light and 12-hour-dark cycle. After 10 days of adaptation, all rats consumed alcohol for four days. In this study, ethanol was administered by intragastric gavage according to Maynard and Leasure’s paradigm (17). The rats were gavaged with ethanol (25% w/v ethanol in Entera Meal supplement with high protein) every eight hours for four days, starting from the first day of the experiment (totally 12 doses). The initial dose for each animal was 5 g/kg. Further doses were determined based on a six-point behavioral intoxication scale (0 = normal; 1 = hypoactive; 2 = ataxia or imbalance and motor inconsistency; 3 = ataxia + dragging abdomen and/or delayed righting reflex; 4 = absent righting reflex; and 5 = absent eye blink reflex). Each point on the scale corresponded to an accompanying dose of ethanol, such that the greater the observed behavioral intoxication, the smaller the subsequent dose. The rats were then randomly assigned into 5 groups of 8 for alcohol withdrawal for seven days including (1) control, (2) curcumin consumption, (3) swimming training, (4) curcumin consumption with swimming training, and (5) sham (using curcumin solvent). Rats in groups 3 and 4 performed swimming training five sessions per week for two weeks at a certain hour of the day (11:00 A.M.). Rats in groups 2 and 4 received the intraperitoneal administration of curcumin, 50 mg/kg, five times weekly for two weeks. The rats in the sham group received the intraperitoneal administration of DMSO (dimethyl sulfoxide) 50 mg/kg, five times weekly for two weeks. Nevertheless, rats in the control group did not perform any training and did not receive any administration. The swimming training protocol, which consisted of 10 sessions, started at 20 minutes and reached an hour after three sessions. It, then, lasted for an hour in the next seven sessions. After each swimming training session, the animals were dried with a towel and returned to their cages (18). The factory-made curcumin (batch No., 820354, Merck, German) was dissolved with DMSO solvent at 10% concentration and injected intraperitoneally with a specific dose 10 times per week for two weeks. At the end of the two weeks, the rats were anesthetized with ketamine and xylazine, and surgically treated to remove the heart tissue. Protein levels of GPX and SOD were removed by Western blot using an American Abcam-made GPX receptor antibody. For Western blotting, equal amounts of protein were isolated by 12% SDS-PAGE polyacrylamide gel. After the electrophoresis, the gel proteins were transferred to PVDF paper and the paper was placed in a blotting solution for an hour. The paper was then placed in the original antibody at 4°C overnight. On the second day, it was washed three times with TBST solution. Then, a paper with beta-actin antibody was placed on the paper and again, it was incubated with the secondary antibody. The beta-actin control was also displayed in the radiology film, and the obtained bands were quantified by the Image J program. For analysis of the research findings, the Kolmogorov-Smirnov test and two-way analysis of variance test were used (P ≤ 0.05).
4. Results
The mean and standard deviation of SOD and GPX protein levels are presented in Table 1. The results in Table 2 show that curcumin consumption (F = 0.75, P = 0.10, and effect size = 0.009) and swimming training (F = 0.66, P = 0.19, and effect size = 0.017) had no significant effect on the increase of SOD protein levels of rats after binge consumption of alcohol. Moreover, swimming training with curcumin consumption (F = 0.21, P = 0.73, and effect size = 0.13) had no interactive effect on the increase of SOD protein levels (Table 2). The results in Table 2 show that curcumin consumption (F = 32.90, P = 0.001, and effect size = 0.74) and swimming training (F = 14.85, P = 0.003, and effect size = 0.57) had significant effects on the increase of GPX protein levels in rats after binge consumption of alcohol. In addition, swimming training with curcumin consumption (F = 10.31, P = 0.008, and effect size = 0.48) had an interactive effect on the increase of GPX protein levels (Table 2). As the effect size of swimming training (0.57) was lower than that of curcumin consumption (0.74), it could be noted that curcumin consumption had more effects on the increase of GPX protein levels than swimming training, and swimming training with curcumin consumption weakened the effect of curcumin.
| Group | SOD (U/mL) | GPX (U/mL) |
|---|---|---|
| Control | 1.15 ± 0.369 | 0.88 ± 0.08 |
| Curcumin | 0.74 ± 0.11 | 1.01 ± 0.07 |
| Swimming training | 0.58 ± 0.13 | 0.58 ± 0.13 |
| Swimming training and curcumin | 1.93 ± 1.003 | 0.98 ± 0.07 |
| Sham | 4.82 ± 4.78 | 0.91 ± 0.002 |
Abbreviations: GPX, glutathione peroxidase; SOD, superoxide dismutase.
Abbreviations: df, degree of freedom; GPX, glutathione peroxidase; SOD, superoxide dismutase.
a Significance level at P ≤ 0.05.
5. Discussion
The results showed that curcumin had no significant effect on the increase of SOD protein levels in rats, but increased GPX protein levels in rats during the withdrawal of binge alcohol consumption. Research on humans and animals has shown that alcohol consumption can lead to severe damage to the heart tissue by reducing the antioxidant factors such as CAT, SOD, and GPX, consequently increasing oxidative stress (19). It has been shown that the binge consumption of alcohol in rats can increase malondialdehyde, increase lipid peroxidation, and decrease GPX and SOD in the heart tissue (20). On the other hand, the protective effects of medicinal herbs such as turmeric and its effective ingredients, such as curcumin, have been shown (21). Some studies examined the effects of turmeric and curcumin on antioxidants and oxidative stress; for example, taking 30 mg/kg of curcumin three days a week for eight weeks had a significant effect on the increase of SOD and GPX in the liver tissue of male rats (7). Moreover, three days a week consumption of 30 mg/kg curcumin for eight weeks had a significant effect on SOD increase and MDA reduction in the kidney tissue of male rats (22). Among the reasons for consistency with the present study, we can point to the same dose used in the two studies, as well as employing the same measurement method in the present study and the same protein levels of variables in the kidney tissue. The consumption of curcumin in the short-term before the competition (70 mg/kg) and in the mid-term one day before the competition (140 mg/kg), could increase the GPX of the young taekwondo athletes but did not have a significant effect on their CAT increase and MDA reduction (23). The antioxidant activity of curcumin is more due to the phenoxy structure and conjugated dual bonds that can readily trap free radicals such as hydroxyl radicals to eliminate them. Curcumin, in addition to the direct removal of free radicals and inhibition of oxidative enzymes such as cytochrome P-450, can increase the activity of intracellular enzymes such as GPX, SOD, and CAT, which have antioxidant properties (24). The results showed that swimming training has no significant effect on the increase of SOD protein levels of rats during the withdrawal of binge alcohol consumption, but significantly increased the GPX protein levels. In line with the present study, the researchers showed that 12 months, three sessions per week of regular aerobic training on the ergometer bike increased the serum levels of SOD, GPX, and glutathione reductase (GR) in the elderly and young people. In addition, the antioxidant increase was significantly higher in young people than in the elderly (25). On the other hand, 12 weeks, five sessions per week and each session of resistance training with an intensity of 70% of one maximum repetition did not significantly change the levels of malondialdehyde in male rats (26). Among the reasons for incompatibility of the study with the present study, the difference in the type of exercise and the basic levels of measured variables can be mentioned. The results of this study showed that swimming training with curcumin consumption did not have interactive effects on increasing SOD protein levels in rats during the withdrawal of binge alcohol consumption, but swimming training with curcumin had interactive effects on increased GPX protein levels. In the present study, the effect size of swimming training was 0.57, which was lower than the effect size of curcumin consumption (0.74). It appears that swimming training had lower effects on the increase of GPX protein levels than curcumin consumption. Nevertheless, the effect size of the interaction of swimming training with curcumin consumption was 0.48. Thus, it can be concluded that simultaneous curcumin consumption and swimming training reduced or weakened the effects of curcumin consumption (on the increase of GPX). It is reported that curcumin is important for collecting and neutralizing free radicals and inhibiting oxidative enzymes such as cytochrome P-450. Moreover, curcumin increases the expression of intracellular glutathione and, by binding to iron, can induce its antioxidant effects. It also acts as a protective agent against oxidative stress by inducing the heme oxygenase enzyme (24). In explaining the main mechanisms responsible for the development of total antioxidant capacity, it can be said that regular exercise by adjusting and modulating the synthesis of both enzymatic antioxidants (GPX, SOD, and catalase) and non-enzymatic antioxidants (uric acid, albumin, and serovaplamin) in muscle cells, cardiac cells and other organs improves the total antioxidant capacity (27). Nevertheless, in the present study, swimming training weakened the effects of curcumin consumption on the increase of GPX. In line with the present study, the researchers stated that a combination of curcumin and low-intensity resistance training had a significant effect on SOD increase and MDA reduction in the kidney tissue of male rats (22). Curcumin supplementation, especially the combination of curcumin with light resistance training, reduced the oxidative stress caused by extreme endurance activity (28). However, eight weeks of curcumin and swimming exercise did not have a significant effect on the expression of CAT and SOD antioxidants in the liver tissue of male rats (7). This study is consistent with the current study but is not in agreement with the increase of other antioxidants. The possible reason for this difference is the difference in the measurements, i.e., in the present study, protein levels were measured while in the above study, the levels of gene expression were measured with the real-time PCR. The small number of statistical samples compared to statistical analyses, the insignificant effect of the intervention on the increase of SOD, and the low number of similar studies for comparison with the present study can be considered as some limitations of the present study. Considering the results of this study, it is suggested for further studies to investigate the effect of training type (endurance and/or resistance training with moderate to low intensity) and its combination with curcumin supplementation on antioxidant factors in rats during withdrawal of binge alcohol consumption.
5.1. Conclusions
In general, it can be concluded that swimming training along with curcumin supplementation has antioxidant effects on the heart tissue of rats during withdrawal of binge alcohol consumption, although this increase was not significant in superoxide dismutase.