1. Background
A considerable incidence, weak prognosis, and limited treatment strategies make GC a major health problem (1). The trend of GC is increasing with a mild trend among the Iranian military population (2). The cancer risk factors and pattern in military population may differ from the general population (3). The effective treatment of GC is needed for early diagnostic strategies (4). Thus, people at risk for developing GC in the military population as well as their families must be detected. The identification of GC risk factors can be effective in screening, early diagnosis, and treatment.
GC is a multifactorial disease that results from the interaction of genetic sensitivity and environmental factors (5). GC has a higher incidence rate in males and older populations (6). Multiple factors such as race (7, 8), Helicobacter pylori infection (9), gastro-esophageal reflux disease (10), dietary factors such as salty (11) and smoked food (12), low consumption of fruits and vegetables (11), smoking (13), family history (14), low socioeconomic groups (15), and blood type A (16) are thought to play a role in the incidence of GC.
Various studies have been mentioned in the genetic predisposition as an important factor in the etiology of GC (17). X-ray repair cross-complementing group 3 (XRCC3) is one of the DNA repair genes, and a sequence variation exists in exon 7 of this gene, which leads to an amino acid change (threonine with methionine) in codon 241 (17, 18). This polymorphism is mentioned as Thr241Met. Several studies have evaluated the association between Thr241Met polymorphisms and GC susceptibility (17, 19-21). There are controversies and diversities in the effect of Thr241Met polymorphism on GC, especially in Asian countries. For example, this polymorphism was not associated with GC risk in the Kashmiri population (22), while it was connected to GC in the Chinese population (23).
2. Objectives
Therefore, having a small study from Iran may enhance the information in regards to the impact of Thr241Met polymorphism on GC in conjunction with environmental factors. Furthermore, there are very few studies on GC in the Iranian military population. Thus, the objective of the current study is surveying the relation between some demographic and environmental factors and Thr241Met polymorphism with GC risk in the Iranian military population.
3. Methods
The present study was a pilot case-control study. Investigated samples were 53 gastrectomy samples of patients with GC from the Imam Reza (501) hospital of Iran army and 91 blood samples from age-sex matched healthy controls. Control subjects were selected from among those who were referred to the 501 hospital, and were free from any type of cancer. Age and gender rate of the cases were considered, and the control group was selected to be in the age range and gender rate of the patients.
Data for cases were obtained from patients registered files and data for controls were collected through questionnaires. Genomic DNA was extracted by SinaClon company DNA extraction kits (EX6041 for tissue and EX6001 for blood), according to the manufacturer's instructions. The extracted DNA was stored at -20ºC until subsequent experiments.
The Thr241Met polymorphism was genotyped by polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP). Primers of 5’-GCTGTCTCGGGGCATGGCTC-3’ (forward) and 5’-ACGAGCTCAGGGGTGCAACC-3’ (reverse) were used to amplify a 208-bp fragment of XRCC3 gene. The amplification cycle was 95ºC for 5 minutes, 30 cycles at 95ºC for 30 seconds, 59ºC for 35 seconds, and 72ºC for 1 minute, and final extension for 10 minutes at 72ºC (17). Amplification of 208-bp fragment was confirmed by electrophoresis on 2% agarose gel. Then, eight microliters of PCR product overnight digested with two units of Nla III restriction endonuclease at 37ºC and separated on 2.5% agarose gel stained with ethidium bromide. Thr/Thr homozygotes presents one fragment of 208 bp, Thr/Met heterozygotes presents three fragments of 208, 120, and 88 bp and Met/Met homozygotes presents two fragments of 120 and 88 bp on the gel image.
The results of Thr241Met polymorphism and demographic and environmental information were analyzed by the SAS 9.3.1 software (24) LOGISTIC procedure to determine the factors affecting GC risk. Associations are assessed using odds ratios (OR) and 95% confidence intervals (CIs).
4. Results
Characteristics of the age and sex ratios for participants are summarized in Table 1. The ratio of males was higher than females in both cases and controls. Also, the mean, maximum, and minimum age were similar for cases and controls. Statistical analysis was performed for age and sex and non-significant differentiation observed for age (P = 0.64 OR = 0.99 (95% CI: 0.96 - 1.03)) and sex (P = 0.92 OR = 1.04 (95% CI: 0.49 - 2.19)) between cases and controls.
| Population | Number | Age | Sex, No. (%) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Minimum | Maximum | Men | Women | ||
| Case | 53 | 65.06 ± 11.53 | 42 | 86 | 34 (64.15) | 19 (35.85) |
| Control | 91 | 64.25 ± 9.21 | 43 | 86 | 58 (63.74) | 33 (36.26) |
| Total | 144 | 64.55 ± 10.10 | 42 | 86 | 92 (63.89) | 52 (36.11) |
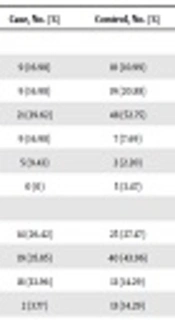
Statistical properties of surveyed factors and OR and P value for GC occurrence are presented in Table 2. As shown in Table 2, the results of the present study indicated that the effects of birthplace and smoking (P = 0.08) on GC were not significant. However the percentage of smokers among patients was higher than healthy individuals, and OR for smoking was high (OR = 3.03).
| Factors | Case, No. (%) | Control, No. (%) | OR (95% CI) | Chi-Square | P Value |
|---|---|---|---|---|---|
| Birth state | |||||
| North | 9 (16.98) | 10 (10.99) | 1 (referent) | ||
| Northwest | 9 (16.98) | 19 (20.88) | 0.54 (0.15 - 0.20) | 0.83 | 0.36 |
| Center | 21 (39.62) | 48 (52.75) | 0.41 (0.13 - 1.29) | 2.32 | 0.12 |
| West | 9 (16.98) | 7 (7.69) | 1.28 (0.31 - 5.32) | 0.12 | 0.73 |
| South | 5 (9.43) | 2 (2.20) | 3.49 (0.37 - 32.93) | 1.20 | 0.27 |
| East | 0 (0) | 5 (3.47) | - | - | - |
| Blood type | |||||
| O | 14 (26.42) | 25 (27.47) | 1 (referent) | ||
| A | 19 (35.85) | 40 (43.96) | 1.33 (0.50 - 3.55) | 0.33 | 0.56 |
| B | 18 (33.96) | 13 (14.29) | 2.99 (1.02 - 8.79) | 3.98 | 0.04a |
| AB | 2 (3.77) | 13 (14.29) | 0.31 (0.04 - 2.24) | 1.34 | 0.25 |
| Cardiovascular diseases or hypertension | |||||
| No | 39 (73.58) | 58 (63.74) | 1 (referent) | ||
| Yes | 14 (26.42) | 33 (36.26) | 0.63 (0.26 - 1.53) | 1.06 | 0.30 |
| Smoking | |||||
| No | 40 (75.47) | 78 (85.71) | 1 (referent) | ||
| Yes | 13 (24.53) | 13 (14.29) | 2.47 (0.89 - 6.87) | 3.03 | 0.08 |
| Family history | |||||
| No | 43 (81.13) | 80 (87.91) | 1 (referent) | ||
| Yes | 10 (18.87) | 11 (12.09) | 2.25 (0.79 - 6.42) | 2.29 | 0.13 |
| XRCC3 (Thr241Met) | |||||
| Thr\Thr | 37 (69.81) | 48 (52.75) | 1 (referent) | ||
| Thr\Met | 16 (30.19) | 41 (45.05) | 0.40 (0.17 - 0.95) | 4.36 | 0.04a |
| Met\Met | 0 (0) | 2 (2.20) | - | - | - |
aSignificant effect
The frequency of blood type B was significantly different between cases and controls (P = 0.04) and the incidence of GC was highest in individuals with blood type B (OR = 2.99). The incidence of GC was lowest in individuals with blood type AB (OR = 0.33). Although, the number of individuals with a family history of cancer in cases was higher than controls, non-significant differences were observed between cases and controls for family history (P = 0.13), as well as for cardiovascular disease or hypertension (P = 0.30).
The frequency of heterozygote genotype (Thr\Met) in the controls was higher than cases (P = 0.04). Due to the fact that the Met\Met genotype observed only in the controls and did not exist in cases, it was not possible to calculate OR and chi-square for this genotype.
5. Discussion
The impacts of gender and age on the incidence of GC have been confirmed in numerous studies (11). In the present study, for an accurate study of other risk factors, age and sex ratios were matched between cases and controls. GC is more common in the elderly individuals. In Europe, the average age of GC patients is 62 years (6). It is reported that in the south of Iran the average age of GC patients is 65.5 and 63.7 for males and females, respectively (25). Also, the risk of developing GC in men is more than women (8). Gender differences may be a reflection of physiological differences. Estrogens may prevent from the development of GC (11). On the other hand, environmental and behavior patterns differ for men and women, especially for the military population in Iran, and these patterns can play a positive role in the development of GC.
The present study did not prove a significant relation between birthplaces with GC risk. Studies reported higher occurrence of GC in the north and northwest of Iran (26). Note that in the previous studies, the place of residence has been considered, however, in the current study, the place of birth has been investigated. The high incidence of GC in north and northwest of Iran may be due to environmental factors such as lifestyle and nutrition.
Although the smoking ratio difference between cases and controls marginally was not significant, but based on OR, GC risk in smokers was three times greater than non-smokers. Previous studies reported different results regarding smoking and increased risk of GC. In a meta-analysis study in Asia and Caucasus (27) and a case-control study in India (28) a non-significant relation was observed between smoking and GC. Another meta-analysis study suggested that smoking increased the risk of both cardiac and non-cardiac GC (13). In addition, a case-control study identified smoking as a risk factor for GC (29). Although the exact mechanism of the relationship between smoking and cancer occurrence is unclear, it is likely that the formation of oxygen radicals and increasing cell death induce cancer causing changes in the lining of the stomach, and promote carcinogenesis (30). On the other hand, it has been reported that smoking increases the damage of the stomach and therefore, smokers have high rates of Helicobacter pylori infection and gastrointestinal inflammation (31).
The incidence of GC was high in individuals with blood type B (OR = 2.99). Previous studies mostly introduced blood type A as most sensitive blood type for GC (32). Blood type A was the second sensitive group to GC (OR = 1.33). However, there are studies that reported blood type B as a sensitive blood type for GC. A study among Chinese reported that blood type B associated with an increase of carcinoma and cardiac cancers (33). According to the results of the present study, it seems that the pattern of blood types impact on GC in Iranian military population is different with others, or the interaction between blood types and other factors is responsible for results inconsistency. The military population may have a different pattern for demographic and environmental factors and their impact on cancer development (34).
The previous study reported that people with cardiovascular disease have a low risk for GC due to the use of a series of drugs (35). This issue may be confirmed by low OR (OR = 0.63) obtained in the current study for cardiovascular disease, however, a marginally non-significant relationship was observed between cardiovascular disease and GC risk. It is possible that some genetic, hormonal, and environmental factors reversely affected the process of developing cardiovascular disease and GC.
The incidence of GC in people with a family history of cancer was higher than those without any family history of cancer (OR = 2.29). Having a first-degree relative with a history of GC is a proven risk factor for GC (14). Similarity of genetic susceptibility and lifestyle in families may be a reason of being high risk in individuals with a family history of cancer. In the present study, the history of all cancers is considered (not only history of GC) and this issue may be a reason for a non-significant relationship between family history and GC risk.
The frequency of Met\Met genotype was very low (two individuals in the studied population). The low frequency of Met\Met genotype also reported in previous studies (17, 19). The role of Thr241Met polymorphism in the GC etiology has been studied in several studies and different results have been reported. A case-control study in Chinese reported that this polymorphism may not be associated with GC (17). Cabral et al. (19), reported a significant association between Thr241Met polymorphism and GC. In that study, the frequency of Thr\Met genotype in patients was higher than the control group, which is in contrast with the results of the present study. In the other previous study (21), the frequency of Thr\Met genotype in controls was higher than patients, which is in accordance with the result of the present research. A meta-analysis confirmed that Thr241Met may be a GC risk factor among Asians (36). Insufficient number of samples may affect the results of the present study.
The incidence of GC has an increasing trend among the Iranian military population (2). The military population is a unique subset of the population, and some demographic and environmental factors differ between military and general population (34). Therefore, demographic and environmental factors affect the incidence of GC; thus, identified risk factors can be implemented in early diagnosis of cancer. Earlier stage at diagnosis leads to better prognosis and increased survival rates (37). These suggest that we need to have good screening programs to identify and prevent GC in our military population and their families using the risk factors and markers that are known in the current study and other similar studies.
5.1. Conclusions
Our study indicated a significant effect of Thr241Met polymorphism and blood type B on GC risk in the Iranian military population. We could not prove the statistically significant association of smoking and family history with GC in our study. The limited number of samples is a fundamental problem in our research and the results may be affected by chance. In general, according to confirming the risk factors of GC in this study and other similar studies, it is recommended to screen programs with gastroduodenoscopy and screening GC markers such as Thr241Met polymorphism and blood type to identify high-risk individuals in military population, and thus, prevent from GC occurrence and mortality.