1. Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the intensive care unit (ICU) (1, 2). Survival of patients admitted to the ICU with AF is lower, particularly for those who develop AF in the ICU, requiring specific management strategies (1, 3-5). Patients with AF frequently present to the emergency department (ED) and often require hospitalization, imposing a significant burden on clinical resources (6, 7). They are at an increased risk for neurological, cardiovascular, cognitive, and psychosocial complications, thromboembolic events, reduced quality of life, and prolonged hospital stays (2, 8). Age, sex, and blood electrolyte imbalance independently increase the risk of AF (9-11). However, data on the relationship between AF prognosis and circulating electrolyte levels are scarce and require further research for validation (12-15).
Atrial fibrillation represents a heterogeneous clinical entity, including first-diagnosed, paroxysmal, persistent, long-lasting, and permanent AF (10). These subtypes differ in epidemiology, management, and prognosis, but most AF studies reported in the literature are community-based and consist of small, heterogeneous samples from various AF categories (16, 17). Specifically, paroxysmal AF (PAF) is a subtype of AF that terminates spontaneously or with intervention within 7 days of onset (10). Evidence regarding the relationship between circulating electrolytes and the prognosis of PAF remains limited. Due to its paroxysmal nature, PAF is often overlooked in clinical practice (18), potentially increasing the risk of adverse outcomes such as stroke or heart failure (18). This underscores the importance of investigating the relationship between electrolyte imbalances in PAF and adverse outcomes.
This secondary analysis was conducted to profile circulating electrolyte concentrations in a relatively large group of patients with PAF admitted to the ED.
2. Objectives
The aim was to characterize patients with PAF who are at a higher risk of mortality or readmission. Identifying such patients would aid in recognizing those requiring critical care and preventing adverse outcomes or progression to chronic AF in the ED or ICU. Our hypothesis was that patterns of serum electrolyte concentrations exist among critically ill patients with PAF.
3. Methods
3.1. Data Source
A retrospective cohort study conducted by Tazmini et al. at Diakonhjemmet Hospital, Oslo, Norway, investigated the incidence of electrolyte imbalances in adults presenting to the ED (17). Their study included all patients aged 18 years and older who visited the ED for any reason between January 1, 2010, and December 31, 2015, and had blood electrolyte levels assessed. At this hospital, blood samples are routinely collected shortly after patients arrive at the ED (17). The dataset comprised 62,991 visits by 31,966 patients. Tazmini et al. reported that individuals with electrolyte imbalances had longer hospital stays and were more likely to be readmitted within 30 days of discharge. They recommended that physicians prioritize the diagnosis, monitoring, and treatment of electrolyte imbalances in newly admitted patients.
Tazmini et al. made their de-identified data publicly available by publishing it as supplementary material in their article, which is archived in a public data repository (17, 19). We performed a secondary analysis of these data to explore the relationship between circulating electrolyte concentrations and PAF prognosis. Tazmini et al.'s research received approval from the Regional Committee for Medical and Health Research Ethics South East (17).
Our study adhered to the principles of the Declaration of Helsinki, as we did not conduct direct measurements on participants but instead performed a secondary analysis using open-access anonymized data. We appropriately credited the primary authors and refrained from republishing raw data. The raw data were licensed under a universal (CC0 1.0) public domain dedication license (19). The complete dataset and its dictionary are openly available in the DRYAD public data repository, accessible via the digital object identifier (DOI): 10.5061/dryad.f3h26j3 (19). This dataset was published on May 8, 2019, and was last accessed on March 16, 2024.
3.2. Study Variables
Two outcomes were recorded in the dataset: Time (days) to death and time (days) to readmission, but only for deceased and readmitted patients. No time-varying data were documented for other patients, resulting in death or readmission being included as a binary outcome, which precluded survival analysis. The dataset recorded the ICD-10 diagnosis code for the "main condition" and a list of "other conditions" at discharge under appropriate headings. Patients with the "main" condition coded as I48.0 in ICD-10 were identified as having PAF. Other recorded variables included:
Age (years), sex, serum sodium (sNa; mmol/L), glucose (sGlc; mmol/L), sNa corrected for sGlc, potassium (sK; mmol/L), calcium (sCa; mmol/L), albumin (sAlb; mmol/L), sCa corrected for sAlb, phosphate (sP; mmol/L), magnesium (sMg; mmol/L), plasma free calcium (fCa; mmol/L).
3.3. Analysis
The dataset was screened for duplicate entries and missing data. For patients with multiple entries, one visit was randomly selected for inclusion. Highly correlated variables were examined, and one variable from each correlated pair was omitted if the absolute correlation coefficient exceeded 0.5. A significant portion of sP and sMg values were missing, and no imputation was performed for the missing data.
Electrolyte concentration data were scaled and used for cluster analysis. To determine the optimal number of clusters, an elbow diagram was generated using the subset of patients with complete electrolyte data. The elbow diagram plots the within-cluster sum of squares against the number of clusters, identifying the optimal number of clusters by locating the "elbow" where adding more clusters does not significantly reduce the sum of square values.
Principal component analysis (PCA) was used to create scatter plots with the first two principal components. Cluster analysis was then performed using the k-means algorithm with the number of clusters suggested by the elbow diagram. The k-means algorithm was implemented with the Python library "KmeansWithNulls", which allows for the inclusion of missing values in the electrolyte data, maximizing the available information while accounting for missing data.
Cluster profiling was conducted to characterize each cluster. A t-test was used to compare means of continuous data, and the χ2 test was used to compare percentages of categorical data. The level of significance was set at two-tailed α = 0.05.
Data analysis and visualization were performed using Python version 3.11.5 for Windows, utilizing libraries such as StatsModels, NumPy, Pandas, and Scikit-learn for robust analysis and results visualization.
4. Results
4.1. Study Sample
The dataset included 62,991 visits from 31,966 patients. Using the ICD-10 code for PAF, we identified 520 patients with the "main condition" of PAF. There were no repeated entries in the PAF subset of the dataset. Among these 520 patients, 287 (55.2%) were male. The mean ± SD age was 69.04 ± 14.64 years, with a minimum age of 22 and a maximum age of 102 years.
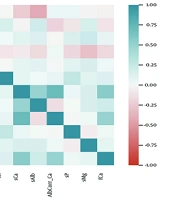
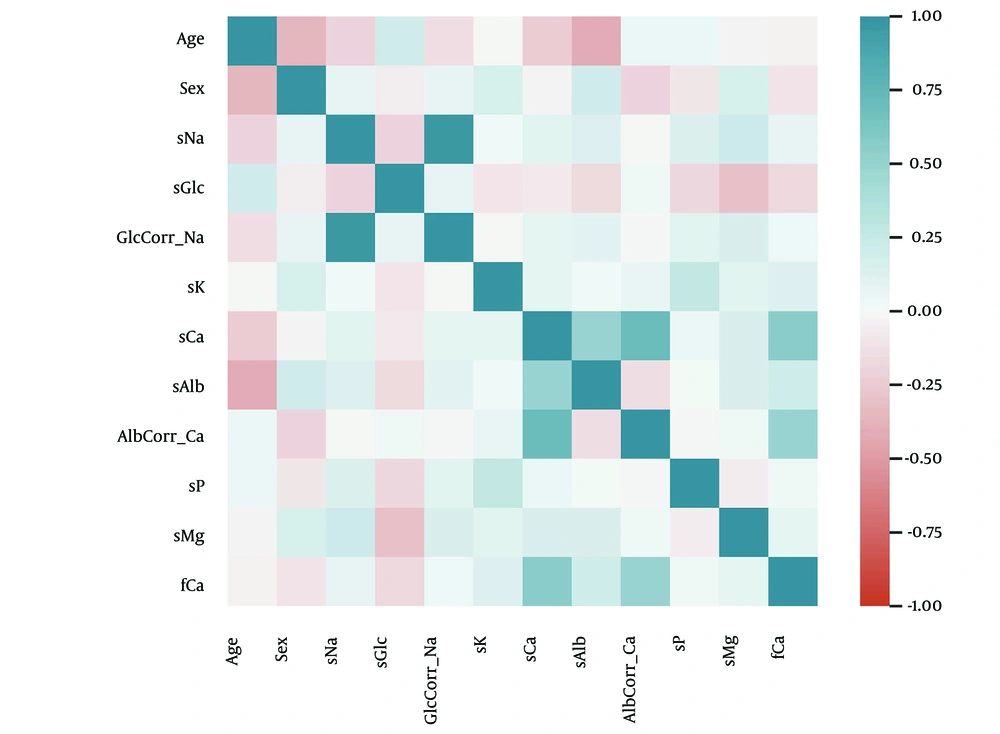
We assessed linear relationships between pairs of study variables using Pearson’s correlation coefficient (r). Significant correlations (r ≥ 0.5) were observed between:
Serum sodium and glccorrected Na (r = 0.97, P < 0.001), sCa and albcorrected Ca (r = 0.71, P < 0.001), sCa and fCa (r = 0.55, P < 0.001), sCa and sAlb (r = 0.50, P < 0.001). The pattern of linear relationships is presented in Figure 1. Based on these correlations, Glc-corrected Na, Alb-corrected Ca, fCa, and sAlb were excluded from further analysis to avoid redundancy.
The characteristics of the analytic sample and the patterns of missing data are shown in Table 1.
| Characteristics | PAF (N = 520) | Missing (%) |
|---|---|---|
| sNA (mmol/L) | 140.21 ± 3.03 | 0.00 |
| sGlc (mmol/L) | 6.62 ± 1.82 | 1.35 |
| sK (mmol/L) | 4.25 ± 0.39 | 0.19 |
| sCa (mmol/L) | 2.35 ± 0.11 | 1.35 |
| sP (mmol/L) | 1.04 ± 0.21 | 79.42 |
| sMg (mmol/L) | 0.83 ± 0.07 | 72.12 |
Abbreviations: sNa, serum sodium; sGlc, serum glucose, sK, serum potassium; sCa, serum calcium; sP, serum phosphate; sMg, serum magnesium.
A significant proportion of data was missing for sP and sMg. The minimum and maximum ages of patients were 22 and 102 years, respectively.
4.2. Clustering
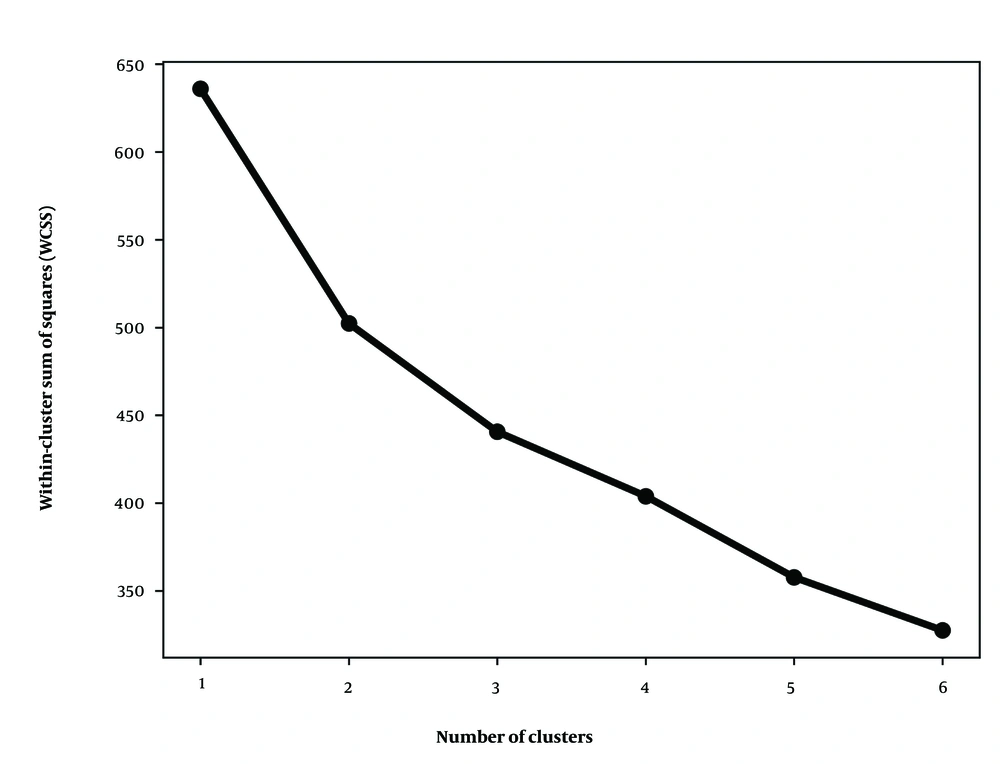
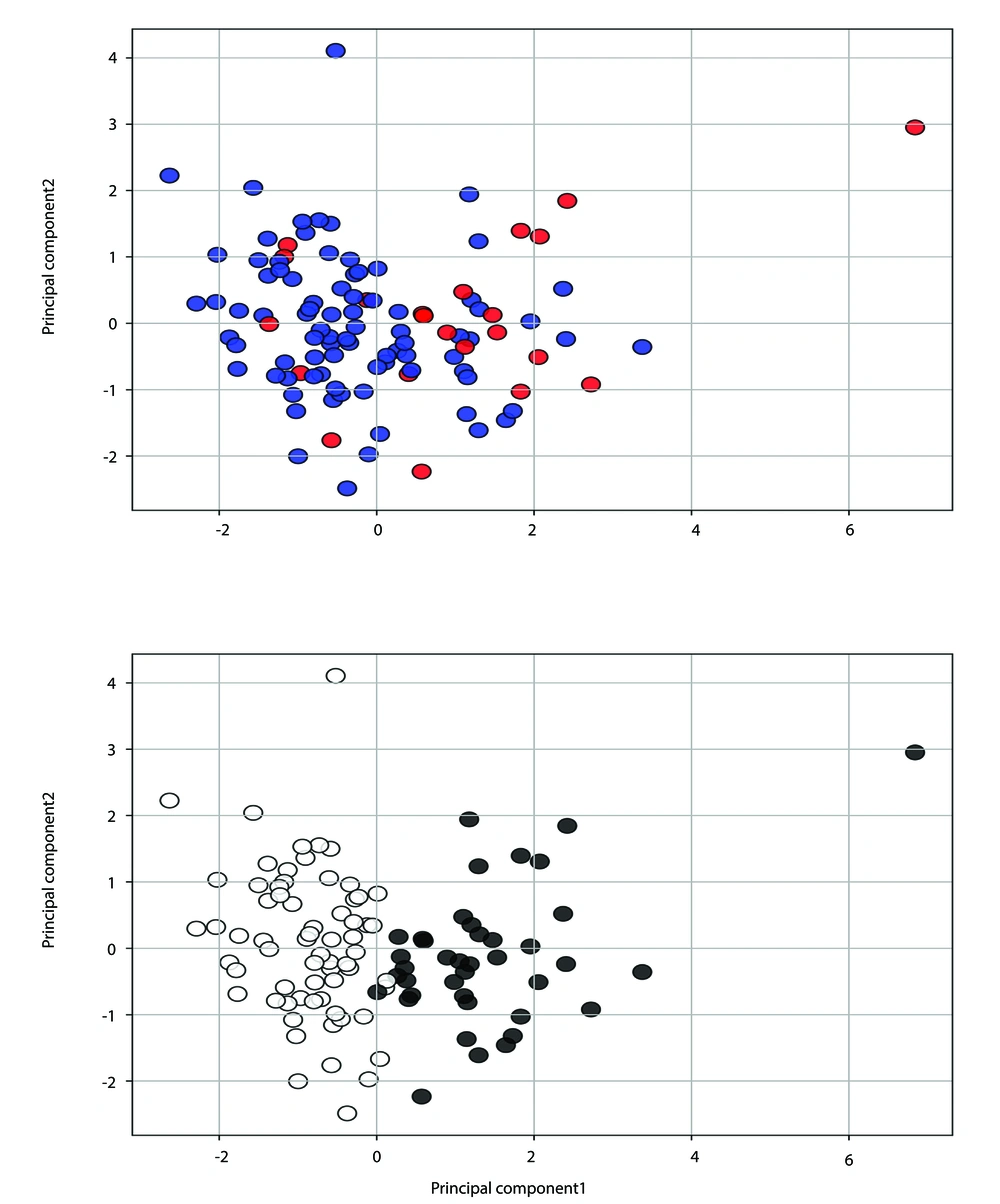
We first assessed the clustering tendency of the electrolyte data using rows without missing variables. Figure 2 presents the elbow diagram, indicating that K = 2 is an optimal choice for the number of clusters in the k-means clustering algorithm. Additionally, the scatter diagram in Figure 3 (upper panel) shows patients with complete data using the first two principal components, where dead or readmitted patients predominantly cluster on the right side. Figure 3 (lower panel) depicts the scatter diagram of the two identified clusters with minimal overlap, suggesting that the clusters differ in their rate of poor outcomes.
Upper panel: The scatter diagram of patients with complete data (N = 106) using the two main principal components. Red solid circles represent deceased or readmitted patients. Lower panel: The scatter diagram of patients with complete data (N = 106) showing two clusters (white and black solid circles).
These initial analyses of complete data suggest that two clusters can be identified in patients with PAF based on electrolyte data, and these clusters may hold prognostic significance.
We then performed 2-means clustering on the complete dataset (N = 520), including missing values. Missing values were not imputed, allowing the clustering algorithm to leverage available data effectively.
4.3. Cluster Profiling
Table 2 shows the results of clustering patients into two groups, including missing values. The two clusters were significantly different in the combined outcome of death or readmission: Cluster I, 57 (35.0%) patients versus cluster II, 66 (18.5%) patients, χ2 (1) = 15.93, P < 0.001, and in the percentage of death: 33 (20.2%) versus 34 (9.5%), χ2 (1) = 10.53, P = 0.001. Our investigations showed that patients in cluster I (high-risk) were older than those in cluster II (low-risk), 74.4 (12.8) versus 66.6 (14.7) years, t = 5.83, P < 0.001, effect size = 0.55. There was no statistically significant difference between the two clusters in the female sex ratio, 81 (49.7%) versus 152 (42.6%), χ2 (1) = 2.01, P = 0.156. Comparisons of the variable “other conditions” between the two clusters showed that patients in the high-risk cluster had higher percentages of endocrine illnesses [34 (20.9%) vs. 19 (5.3%), χ2 (1) = 27.84, P < 0.001] and cardiovascular illnesses [59 (36.2%) vs. 79 (22.1%), χ2 = 10.65, P = 0.001]. Overall, patients in the high-risk cluster were older, had higher levels of sGlc, and higher ratios of endocrine and cardiovascular comorbidities. They showed lower concentrations of sNa, sK, sCa, sP, and sMg.
| Characteristic | Cluster I (N = 163) | Cluster II (N = 357) | t-Statistic | P a | Cohen's d | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Missing (%) | Mean ± SD | Missing (%) | ||||
| sNA (mmol/L) | 137.72 ± 3.20 | 0.00 | 141.35 ± 2.12 | 0.00 | -15.29 | < 0.001 | -1.44 |
| sGlc (mmol/L) | 8.01 ± 2.44 | 0.61 | 5.98 ± 0.88 | 1.68 | 13.71 | < 0.001 | 1.30 |
| sK (mmol/L) | 4.12 ± 0.42 | 0.61 | 4.3 ± 0.37 | 0.00 | -4.76 | < 0.001 | -0.46 |
| sCa (mmol/L) | 2.28 ± 0.12 | 0.61 | 2.38 ± 0.09 | 1.68 | -10.48 | < 0.001 | -0.99 |
| sP (mmol/L) | 0.95 ± 0.16 | 76.07 | 1.1 ± 0.22 | 80.95 | -3.71 | < 0.001 | -0.74 |
| sMg (mmol/L) | 0.79 ± 0.07 | 70.55 | 0.84 ± 0.06 | 72.82 | -3.99 | < 0.001 | -0.70 |
Abbreviations: sNa, serum sodium; sGlc, serum glucose, sK, serum potassium; sCa, serum calcium; sP, serum phosphate; sMg, serum magnesium.
a Significant at P < 0.05.
5. Discussion
This study was conducted to identify prognostic clusters of patients with PAF using circulating electrolyte concentrations. Our results showed that there are at least two clusters of patients with PAF that differ in the risk of death or readmission. We applied a well-established unsupervised machine learning algorithm to develop a model for clustering PAF using electrolyte concentrations. The clustering scheme identified a high-risk group of patients characterized by older age, higher mean sGlc, and lower concentrations of sNa, sK, sCa, sP, and sMg. Endocrine and circulatory comorbidities were also more common in the high-risk cluster. Despite the large variance inherent in the study problem and the community setting in which the data were collected, the study results can be referenced based on electrolyte profiling. Our results did not show a significant difference in sex ratio between the two clusters. These results provide insight into the susceptibility of patients with PAF to experience poor outcomes.
Clustering of patients with PAF based on serum electrolyte concentrations has not been reported in the literature. However, some of our findings are consistent with previous studies suggesting that electrolyte disturbances may play a role in the pathogenesis of AF (12, 15). The incidence of AF increases in the general population with advancing age (20, 21). The association between age and poor prognosis may be attributed to the longer time frame for risk factors to induce structural changes, as well as the relatively indistinct age-related changes in cellular electrophysiology (20).
Studies have suggested differences in the epidemiology of PAF between men and women (22, 23). In general, PAF is more frequent in male patients (23). However, women with PAF are less likely than men to have frequent attacks (22), and they experience less progression of the arrhythmia compared to men (23). These findings align with our results, as we observed a higher proportion of men in our sample. However, the sex ratio was not significantly different between the two clusters in our study. The relationship of other risk factors may have compensated for the effects of the different PAF prevalence between men and women on experiencing poor outcomes.
Patients with diabetes mellitus are at a higher risk of death and cardiovascular events compared to the general population (24). Specifically, diabetic patients with PAF exhibit significantly impaired left atrial deformation patterns, characterized by decreased left atrial strains and increased stiffness (25). Additionally, patients with coexisting type 2 diabetes mellitus and PAF are at increased risk of left ventricular functional impairment and hyperuricemia (26, 27). Our study plausibly reflected these findings, as the high-risk cluster demonstrated a higher mean sGlc concentration.
A comprehensive review has shown that remodeling of Ca, Na, and K channels can influence ion channel protein expression, fibrosis development, gene transcription, and ion channel redistribution, all of which may contribute to the persistence of AF (15). Meanwhile, the relationship between electrolyte concentrations and selected arrhythmias is complex (12). Low sNa concentrations are associated with an increased risk of AF. For example, one study reported that mean sNa was significantly lower in an AF group compared to a control group [136.0 (18.3) mEq/L vs. 142.0 (23.9) mEq/L, P = 0.04] (28). Similarly, another study found that hyponatremia (serum sodium < 135 mmol/L) was associated with a higher risk of all-cause mortality within 365 days post-discharge in patients with AF without heart failure (HR 1.55, 95% CI: 1.05 - 2.28) (29). These findings align with our results.
Research results do not directly address the relationship between sCa and the risk of AF. Instead, they focus on the role of parathyroid hormone and related proteins that influence Ca homeostasis, rather than the direct effects of sCa concentration (30, 31). However, studies have reported that the top quintiles of sMg, sK, and sP had a lower AF prevalence compared to those in the bottom quintiles (12). Elevated sMg levels have also been found to decrease the likelihood of AF after cardiac surgery (15). A study of the Framingham community suggested that low sMg is moderately associated with AF in individuals without cardiovascular disease (13). Consistent with these findings, our results suggest that higher serum electrolyte concentrations are associated with more favorable outcomes in patients with PAF.
Differences between our study and previous research should be considered when interpreting the results. Previous studies have not specifically included individuals presenting to the ED as part of their target population. Medical conditions such as hypertension, diabetes mellitus, chronic kidney disease, and congestive heart failure are common in critically ill patients and can profoundly affect electrolyte balance through impacts on renal function and hormonal regulation (32). Additionally, none of the relevant published studies have focused exclusively on the PAF subtype of AF.
Our approach to data analysis also differs. Some studies did not provide a detailed report of missing data, while others did not employ clustering analysis. Logistic regression has often been used for supervised data classification without thoroughly examining its underlying statistical assumptions. Patients presenting to the ED or ICU frequently receive multidrug regimens, including diuretics, antihypertensives, and antiarrhythmics, all of which can affect electrolyte balance and cardiac electrophysiology.
Our study included data from a non-specific sample of patients with PAF presenting to the ED rather than the general population. We identified two distinct clusters with different prognoses in patients with PAF, emphasizing the need for ongoing investigations of PAF in the ED or ICU settings.
Overall, AF is a common occurrence in critically ill patients admitted to the ICU and ED (33). Managing the risk factors for AF helps prevent the development of the arrhythmia, improves the treatment process, and provides a better prognosis for patients (34). Our investigation identified two distinct clinical subgroups of patients, each with a different prognostic outlook. Integrating electrolyte assessment into standard clinical practice shows promise for improving the identification of individuals with PAF who are at an increased risk of adverse outcomes.
We analyzed a large cohort of patients with diverse health profiles. Using a sophisticated algorithm, we developed a study model capable of handling missing data, enabling us to extract insights from the available evidence without resorting to data imputation. This approach is particularly relevant for variables like sMg and sP, where missing data could otherwise lead to erroneous conclusions if synthetic data were used. The developed model can be integrated into decision-support frameworks to automate the identification of patients at an increased risk for mortality or readmission. This promotes targeted interventions aimed at managing potential adverse outcomes of PAF.
Our findings are particularly relevant for patients admitted to the ICU, where AF is the most common form of cardiac arrhythmia.
5.1. Strengths and Limitations
We included a substantial cohort of patients diagnosed exclusively with PAF, all of whom were admitted to the ED of a single hospital. However, the single-center nature of our study may limit the generalizability of our findings to broader populations. Time to mortality or readmission was documented only for patients with these events, precluding the assessment of PAF incidence trends, examination of correlated risk factors over time, and calculation of hazard ratios due to the lack of temporal data for all patients.
A key strength of the study was the reliance on objective laboratory assessments, minimizing variability across different nationalities. With larger sample sizes, future research could explore the association between electrolyte concentrations and different types of AF within specific comorbidity subgroups. Additionally, adjustment for treatment effects on PAF incidence could be achieved with a much larger sample.
5.2. Conclusions
Using an unsupervised algorithm, we identified potential subgroups within a large sample of patients with PAF. The algorithm effectively handled missing data without the need for artificial data imputation. Analysis of circulating electrolyte levels revealed two distinct patient clusters with different prognostic outcomes. One high-risk cluster was identified, consisting of older individuals with elevated sGlc levels and decreased serum concentrations of sNa, sK, sCa, sP, and sMg. There was no significant difference in the sex ratio between the clusters. The high-risk cluster also had a higher prevalence of endocrine and cardiovascular comorbidities.
These findings provide valuable insights into PAF outcomes and highlight the importance of integrating electrolyte assessments into routine care to improve AF management. Further research is needed to elucidate the underlying mechanisms and refine clinical management strategies for patients with PAF.