1. Background
Endoscopic retrograde cholangiopancreatography (ERCP) is an advanced procedure used for both diagnosis and therapeutic objectives in patients with pancreaticobiliary disorders (1). Although it is a difficult technique that requires specialized training, the use of ERCP appears to be increasing with time (2). The most frequently encountered important ERCP complications are pancreatitis, bleeding, infection, and perforation (3). An increase in the plasma amylase level is common after doing ERCP, occurring in about 75% of patients, and previous studies have reported that the assessment of plasma amylase and lipase 2 to 4 h after doing ERCP is useful in the forecast of post-ERCP pancreatitis (PEP) (4). Post-ERCP pancreatitis is the most prevalent adverse effect of the ERCP procedure, and its incidence was announced from 4% in low-risk patients up to 40% in high-risk patients (5). Although the pathophysiology of PEP is not clear, PEP is assumed to spread from a pro-inflammatory cascade arising from pancreatic acinar cell lesions, leading to systemic cytokine emancipation (6). In previous studies, a range of different medications has been evaluated for prevention or alleviation of PEP, such as indomethacin/diclofenac (7), epinephrine (8), antibiotics (9), and antioxidants (10).
Oxidative stress has been mentioned as a critical mechanism of PEP. Extravagant reactive oxygen species (ROS) produce inflammation and expansion of PEP through zymogen losing granules, granulocyte moving, tissue necrosis, and elevated amylase and lipase function. It seems that in acute pancreatitis, overstimulation of ROS and the deficiency in the power of radical scavengers cause an increase of ROS in pancreatic tissue (11). CoQ10 is a lipid-soluble quinone in humans, and it has an essential role in the mitochondria as an electron transport. Also, it has been mentioned as an antioxidant in recent decades. These antioxidant activities within the electron transport chain of the mitochondria increase the ability of electron transport, thereby preventing the decrease of uncontrolled electrons. Additionally, they facilitate the recycling of other antioxidants, including vitamin C and work against free radicals or oxidants, reducing their levels and counteracting their harmful effects (12). Different clinical trials have evaluated the role of CoQ10 in the decrease of oxidative stress, reporting significant results in the management of cardiovascular, renal, pulmonary, liver disease, and neurologic diseases (13). In an animal study, Shin et al. reported the defensive role of CoQ10 against acute pancreatitis. They induced a model of acute pancreatitis by injection of cerulein intra-peritoneally or by pancreatic duct ligation in mice. The use of CoQ10 alleviated the pancreatitis intensity, as shown by a decrease in acinar cell death, parenchymal edema, inflammatory cell infiltration, and alveolar thickening in both mice models. Also, the reduction of infiltration of immune cells (including monocytes and neutrophils and augmentation of chemokines, such as CC chemokine-2 and C-X-C chemokine-2 in the pancreas) was shown in the mentioned study. They concluded that CoQ10 could impair pancreatic injury by controlling inflammatory cytokines and inflammatory cell infiltration (14). Mirmalek et al. evaluated the role of CoQ10 on L-arginine-induced acute pancreatitis in a rat model. For the assessment of oxidative stress, they measured pancreatic superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA), and myeloperoxidase (MPO). Also, a histopathological evaluation was done. In a dose-dependent manner, the concentrations of amylase, lipase, MDA, and MPO decreased, while the levels of SOD and GSH increased. Regarding histopathology, there is a protective role for CoQ10. Overall, they concluded that administration of CoQ10 has an amelioration property against pancreatic injury (15).
2. Objectives
Considering the deficiency of data regarding the effect of CoQ10 in the prevention/alleviation of PEP, we conducted a randomized, controlled clinical trial to evaluate the role of prophylactic rectal indomethacin with and without oral CoQ10 for prevention/alleviation of PEP in patients undergoing ERCP.
3. Methods
3.1. Study Design
This clinical randomized, double-blind trial was conducted on patients who underwent ERCP from October 2022 to February 2023 at the endoscopy procedures ward of a teaching and referral hospital in Tehran, Iran. The ethical approval of the trial was obtained from the Shahid Beheshti University of Medical Sciences ethical committee (IR.SBMU.PHARMACY.REC.1401.091). A registered number for clinical trials (IRCT20121021011192N13) was obtained from the Iranian Clinical Trial Registry.
3.2. Patients
All individuals between the ages of 20 and 80 who were eligible for ERCP were included in the trial. Participation in the trial was voluntary, and the patients provided their consent by signing a written informed consent form. Patients with a history of previous biliary/pancreatic sphincterotomy, the need for replacement of pancreatic/biliary stent, creatinine clearance below 50 mL/min, heart block, bradycardia, baseline any of amylase or lipase more than 3 folds the upper normal limit, child C cirrhotic patients, childbearing, breastfeeding mothers, history of allergy to any of indomethacin or CoQ10, and receiving of any drugs that might affect the PEP affair, such as NSAIDs and octreotide, were excluded from the study.
3.3. Intervention
Before randomization and intervention, a medical history and blood specimen were obtained from the participants. Then, the participants were randomly allocated into 2 arms by simple randomization based on computer-generated random numbers. The participants, the gastroenterologist who did ERCP, and the evaluator did not recognize to which arm a patient would be allocated before that patient entered the trial, and allocation concealment was kept. All patients received indomethacin (100 mg) rectally just before doing ERCP. The intervention and control arms received 2 tablets of CoQ10 (100 mg; total, 200 mg) orally or 2 tablets of CoQ10 placebo 2 h before doing ERCP. The CoQ10 tablets were prepared by Jalinous Pharmaceutical Company, Iran. Also, indomethacin suppositories were prepared by Behsa Pharmaceutical Company, Iran. Participants were sedated using the same protocol of midazolam and morphine. All ERCP procedures were done by an expert gastroenterologist who had the experience of doing over 100 ERCPs using standard interventional duodenoscopes.
3.4. Outcome Measures
After doing the ERCP, each participant was closely evaluated for the occurrence of PEP. The evaluation was done by an investigator who was blinded to the arm allocation. Amylase and lipase concentrations were determined 6 h after the ending of ERCP in each participant. The definition of PEP was severe abdominal pain with an acute beginning or aggravation, requiring an extended hospital admission of a minimum of 2 days and increased amylase/serum lipase concentrations of more than 3 times above the upper limit of normal. Patients were categorized as mild, moderate, or severe according to the standard guidelines (16). The rate of PEP was evaluated between the 2 arms. All known confounders, such as sex, primary amylase, and lipase, were also compared between the 2 arms. The level of MDA was determined at the baseline and 6 h after ERCP. All potential adverse drug reactions were recorded during the trial. If participants present with any suspected adverse drug reaction, they are instructed to inform the evaluators to provide necessary management.
3.5. Statistical Analysis
The data were analyzed using SPSS version 22 (SPSS Inc, Chicago, IL, USA). Statistical testing is 2-sided, and P values less than 0.05 were considered significant. Quantitative and categorical data were described as mean ± SD and numbers/percentages, respectively. The occurrence and severity of PEP were compared between the 2 arms using the chi-squared test or Fisher’s exact test. Parametric and nonparametric variables were analyzed using the independent 2-sample t-test and Mann-Whitney U test; however, categorical variables were analyzed using the chi-square test or Fisher’s exact test. Based on similar articles regarding the reduction of PEP by the addition of an additional agent to indomethacin (6, 7) demonstrate a decrease in the rates of occurrence of PEP from 15% to 5% because of CoQ10 addition by using an alpha error of 0.05 and a power of 0.80, at least 165 participants were required in each arm. A decrease of 10% in the PEP rate is favorable based on previous trials (7, 8).
4. Results
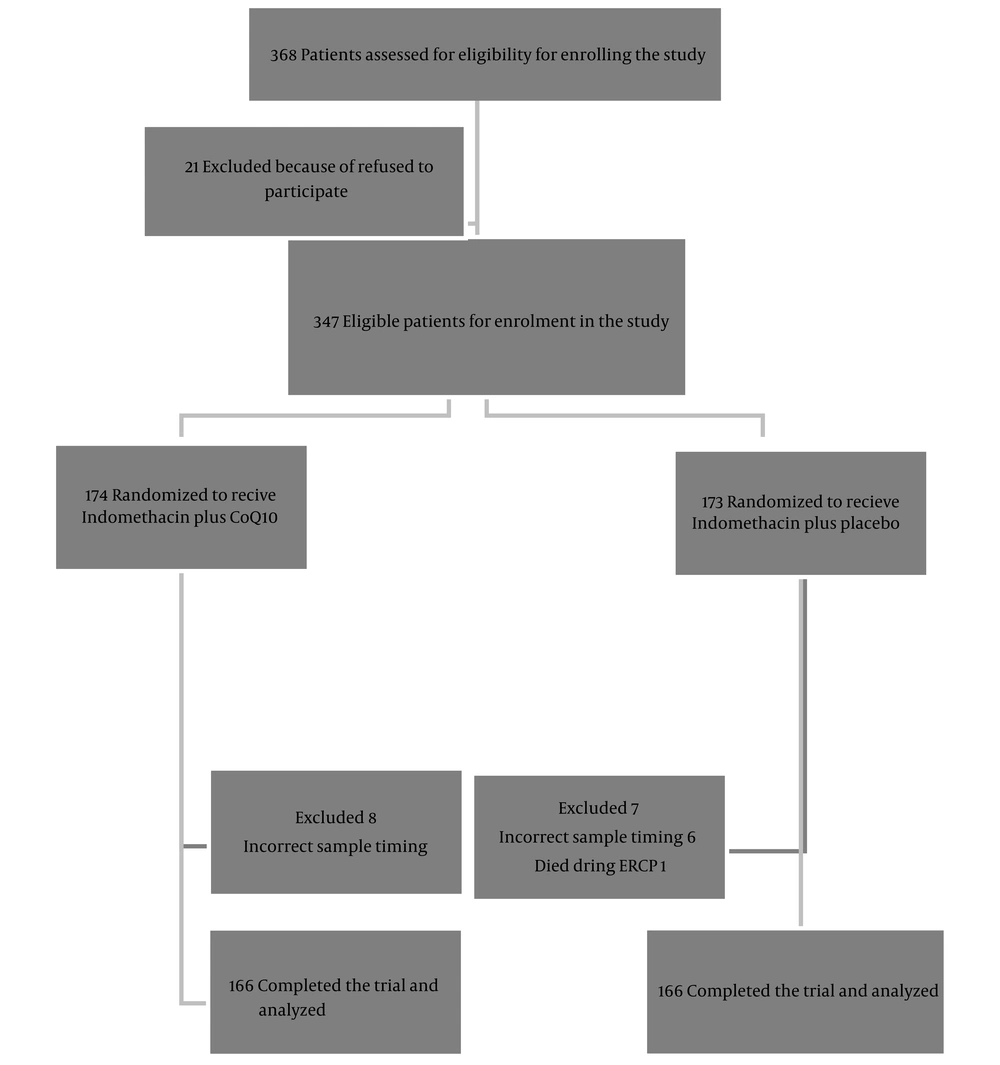
During 4 months (from October 2022 to February 2023), a total of 368 patients underwent ERCP in the mentioned ward. Among 347 eligible patients enrolled in the study, 15 were excluded, and eventually, 332 patients finalized the trial. Administered medications were tolerated well, and there was no dropout in this trial because of drug side effects. The flowchart of patients throughout the study is shown in Figure 1.
Table 1 presents the patient's characteristics, baseline biochemical parameters, and ERCP indications and difficulties.
| Variables | CoQ10 Group (n = 166) | Placebo Group (n = 166) | P-Value |
|---|---|---|---|
| Age (y) | 55.0 ± 13.1 | 58.0 ± 13.4 | 0.733 |
| Females | 92 (55.4) | 87 (52.4) | 0.764 |
| BMI (kg/cm2) | 23.3 ± 3.2 | 22.9 ± 3.4 | 0.689 |
| Alcohol users negative | 158 (95.1) | 157 (93.9) | 0.557 |
| Primary amylase (IU/L | 62 ± 21 | 57 ± 23 | 0.432 |
| Primary lipase (IU/L) | 81 ± 13 | 78 ± 16 | 0.342 |
| Primary MDA (mcmole/L) | 1.41 ± 0.18 | 1.39 ± 0.16 | 0.786 |
| Indications | 0.762 | ||
| CBD stone with or without cholangitis | 126 | 122 | |
| Periampulary tumors | 24 | 29 | |
| Cholangiocarcinoma | 13 | 11 | |
| CBD stricture | 2 | 2 | |
| Parasites | 1 | 2 | |
| Procedure difficulty | 0.336 | ||
| 1 | 34 | 39 | |
| 2 | 109 | 100 | |
| 3 | 15 | 14 | |
| 4 | 8 | 13 |
Abbreviations: MDA, malondialdehyde; BMI, body mass index; CBD, common bile duct stone.
a Values are presented as % or mean ± SD.
ERCP difficulties have been defined based on the American Society of Gastroenterology and Endoscopy (ASGE) grading system. The ASGE ERCP grading scale indicates the procedure's complexity and predicts the chance of complications (16). No significant difference was seen in age, sex, body mass index (BMI), alcohol use, primary amylase, primary lipase, primary MDA, ERCP indication, and ERCP difficulty between the groups. A total of 347 participants were entered in the intention-to-treat (ITT) analysis, and 322 participants were entered in the per-protocol (PP) analysis. Concerning ITT analysis, 42 out of 347 patients and, regarding PP analysis, 31 out of 280 patients had pancreatitis (12.1% and 13%, respectively). Table 2 shows the PEP rate and severity in the 2 arms.
| Intention to Treat | Co Q10 Arm | Placebo Arm | P |
|---|---|---|---|
| Pancreatitis negative | 157 (90.2) | 148 (85.6) | 0.048 |
| Mild pancreatitis | 14 (8.0) | 19 (11) | |
| Pancreatitis positive | |||
| Moderate pancreatitis | 2 (1.2) | 3 (1.7) | |
| Severe pancreatitis | 1 (0.6) | 3 (1.7) | |
| Total | 174 | 173 | |
| Per Protocol | Co Q10 Arm | Placebo Arm | P |
| Pancreatitis negative | 149 (89.8) | 141 (84.9) | 0.043 |
| Mild pancreatitis | 14 (8.4) | 19 (11.4) | |
| pancreatitis positive | |||
| Moderate pancreatitis | 2 (1.2) | 3 (1.8) | |
| Severe pancreatitis | 1 (0.6) | 3 (1.8) | |
| Total | 166 | 166 |
a Values are expressed as No. (%).
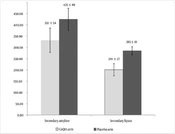
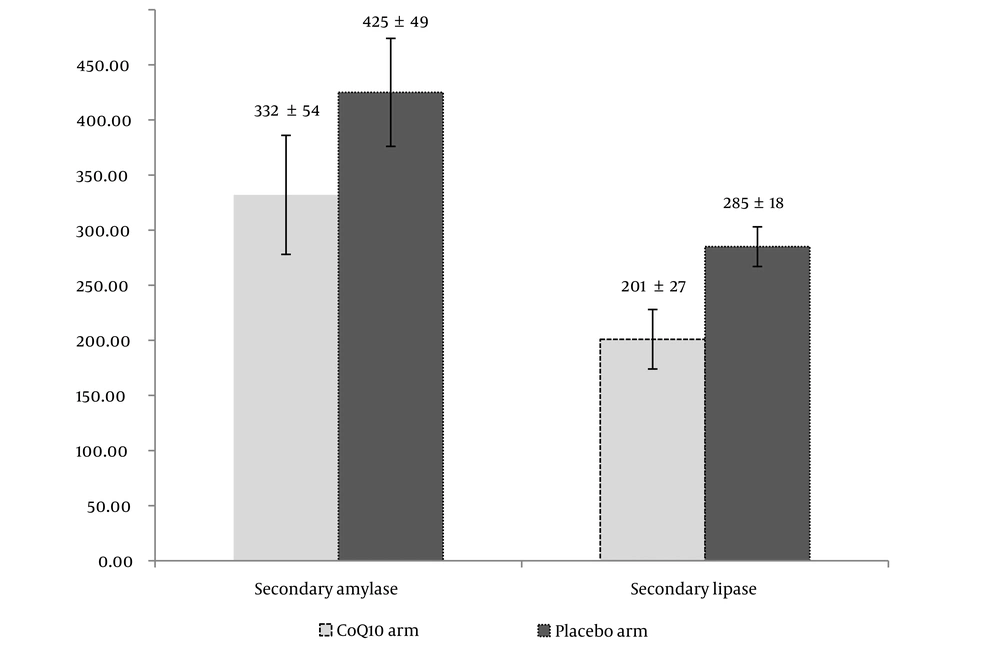
The analysis showed that there was a meaningful difference between the 2 arms based on ITT and PP analyses (P = 0.048 and P = 0.043, respectively). The secondary concentrations of amylase and lipase are shown in Figure 2 in 2 arms distinctively.
The results revealed a significant difference in the secondary concentration of amylase and lipase between the 2 arms compared to the primary concentration (P = 0.038 and 0.042 in CoQ10 and placebo arms, respectively). The secondary amylase level was less in the CoQ10 compared with the placebo group. Such a difference was observed regarding secondary lipase, too. The statistical analysis revealed a significant difference between the secondary amylase and lipase among the 2 groups (P = 0.032 and 0.022, respectively). The results revealed an increase in the amount of MDA after 6 h of ERCP (secondary MDA) in both arms (3.1 ± 0.2 mcmole/L and 3.8 ± 0.4 mcmole/L in CoQ10 and placebo arms, respectively). Although there was a significant increase in secondary MDA levels compared to primary MDA in both the CoQ10 and placebo arms (P = 0.35 and 0.24, respectively), there was a notable difference between the 2 groups specifically in terms of secondary MDA levels (P = 0.036).
5. Discussion
It seems that oxidative stress and imbalance between oxidation processes and antioxidant defenses had an important role in the pathogenesis of a range of human diseases (17). In a meta-analysis, Dai et al. have reviewed the effect of CoQ10 on biomarkers of oxidative stress in humans. They have concluded that administration of CoQ10 at least in a dose of 100-150 mg/day may be effective in alleviating oxidative stress in humans (18). Post-ERCP pancreatitis is a complication whose exact mechanism has not been determined. Damage to the pancreatic acinar cells starts a complex flood of events that contains elevated creation of ROS, resulting in the oxidation of proteins and lipids and upset of the pancreatic membrane (19). Regarding the efficacy of CoQ10 in the alleviation of oxidative stress, we designed the present trial to determine the efficacy and safety of the addition of CoQ10 vs placebo to the rectal indomethacin in the PEP occurrence and severity. To reduce PEP, the guidelines of the European Society of Gastrointestinal Endoscopy (2020) recommend the use of 100 mg of diclofenac or indomethacin just before or after doing ERCP (20). Therefore, to comply with moral interests, the current study was designed to determine whether CoQ10 addition to indomethacin suppository could reduce the occurrence of PEP. In our study, the rate of PEP among the CoQ10 arm was lower than in the placebo arm (10.2% vs 15.1%), and the statistical analysis showed a significant difference in the mentioned values (based on PP analysis, P = 0.043). In the interpretation of the results, confounding factors are important. In a recent review by Cahyadi et al., the risk factors of PEP were evaluated. They referred to some characteristic variables (such as female sex, lower age, obesity, and history of alcohol intake) as the most important risk factors (21). In our trial, both groups were matched concerning sex position, mean age, BMI, and alcohol use. Also, indications of ERCP and difficulty in doing ERCP were not different between the 2 groups (P = 0.762 and 0.336, respectively).
Different biomarkers were used for the assessment of PEP. A serum amylase concentration that is more than 4 to 5 times the upper limit of normal, along with the presence of clinical manifestations of pancreatitis, has been identified as a dependable indicator for diagnosing PEP. However, the exact timing and concentration of serum amylase raises are unknown (22). It has been suggested that a dynamic rise of serum amylase between 3 and 6 hours after ERCP can be diagnostic of PEP (1). We measured the amylase level 6 h after doing ERCP in this trial. Although amylase levels were raised in both groups, the placebo group had a significantly higher level statistically compared with the CoQ10 group (P = 0.032). Similar patterns were observed for the lipase level in our trial, where lipase levels were raised after 6 h in both arms; there was a significant difference between the 2 groups. The lipase level increased significantly in the placebo group vs the CoQ10 group (P = 0.022). According to our results, it seems that a combination of indomethacin and CoQ10 may be effective in controlling the amylase and lipase elevation vs indomethacin alone. Malondialdehyde is one of the ultimate products of polyunsaturated fatty acid peroxidation in cells. An elevation in free radicals leads to overproduction of MDA, and the MDA concentration is considered a biomarker of oxidative stress in humans (23). Abu-Hilal et al. evaluated the MDA concentration in patients with the diagnosis of acute pancreatitis. They measured MDA levels at different times (including 24 h) of the acute pancreatitis onset. They concluded that serum MDA might be a useful biomarker for the estimation of pancreatitis severity in the very early stages of acute pancreatitis (24). Our findings showed that MDA levels increased in both groups 24 h after doing ERCP vs baseline. However, there was a significant difference between the 2 arms regarding secondary MDA. There was a significant increase in secondary MDA levels in the placebo arm compared to the CoQ10 arm (P = 0.036). Although we only measured MDA as an oxidative biomarker, it seems that CoQ10 might be effective in the alleviation of the oxidative stress process. To the best of our knowledge, this study is the first clinical trial to evaluate the effect of CoQ10 plus indomethacin vs indomethacin alone on the PEP rate and severity. We only measured MDA as a significant marker of oxidative stress, and it is strongly recommended to evaluate other biomarkers involved in oxidative stress in future studies.