1. Background
Spinal anesthesia and cesarean delivery are the standard management for women with preeclampsia (PE) in the absence of contraindications to neuraxial anesthesia (1).
Maternal hypotension is a well-known complication of subarachnoid block during cesarean sections. The most common mechanism of hypotension is the dominance of vasodilation secondary to sympathetic nerve fiber blockage at the preganglionic level (2). If not urgently managed, hypotension can have both maternal and fetal consequences, including intraoperative nausea, vomiting, fetal acidosis, and fetal hypoxia (3). An increased risk of end-organ injury was reported when intraoperative hypotensive episodes lasted more than 10 min (4).
Systolic blood pressure (SBP) should be maintained ≥ 90% of the baseline, and intravenous vasopressors are warranted if it decreases to less than 80% of the baseline (5). Patients with PE are reportedly less prone to postspinal hypotension compared to normotensive patients, making a prophylactic infusion not reasonable in this setting, and vasopressors should be used when hypotension occurs (5-7). There is also the concern that pregnant women with PE might be sensitive to exogenous norepinephrine (NE) (8).
The management of postspinal hypotension has long been carried out with phenylephrine. Its main drawbacks are bradycardia and possible maternal cardiac depression. There is a recent focus on the use of NE either as a bolus or intravenous infusion in the management of postspinal hypotension because of its α-adrenergic agonist activity (2, 3, 9-13). No significant difference in the efficacy of NE and phenylephrine has been reported, but NE has the advantage of being associated with less incidence of maternal bradycardia (14).
Unfortunately, limited data exist on the efficacy of the use of NE to treat postspinal hypotensive episodes in females with PE. Likewise, limited data exist on the optimum bolus dose of NE in this setting. Lower or higher doses of NE bolus can lead to failed management or reactive hypertension, respectively. A 4-µg NE dose was recently investigated in preeclamptic mothers (6).
2. Objectives
This study aimed to compare the safety and efficacy of 3 different NE bolus doses (3, 4, and 5 µg) in the management of postspinal hypotension during lower segment cesarean section in patients with PE.
3. Methods
3.1. Ethical Considerations
The study protocol obtained approval from the Research Ethics Committee of Cairo University, Egypt (code: MS-260-2019). Written informed consent was obtained from the study participants after they were informed about the study objectives, methodology, risks, and benefits. The investigators were responsible for keeping the participants’ privacy and security of the data. This clinical trial was registered at ClinicalTrials.gov (ID: NCT05368415; date: May 10, 2022).
3.2. Study Design and Settings
This randomized, double-blind, parallel-group clinical trial took place in the obstetric theatre of Cairo University Hospitals, Egypt, between June and August 2022.
3.3. Eligibility Criteria
The study included full-term pregnant mothers with PE, aged between 18 and 40 years, scheduled for lower segment cesarean delivery who developed postspinal hypotension. Pregnant women with peripartum bleeding, coagulation disorders, chronic hypertension, papilledema, renal or hepatic diseases, or fetal distress were excluded.
3.4. Randomization and Allocation Concealment
A computer-generated sequence was achieved by the principal investigator through an online random number generator. The generated codes for participants were placed into sequentially numbered, opaque envelopes. Each envelope included the instructions for preparing the drug bolus. The envelope was opened by an anesthesia resident (who was not involved in the patient management), and he was responsible for preparing the study drug.
3.5. Intervention
Upon arrival to the operating room, patients were monitored using electrocardiography and pulse oximetry. Blood pressure was also monitored non-invasively using a General Electric (GE, SolarTM 8000i) monitor. Baseline mean arterial pressure (MAP) and heart rate (HR) were recorded while the patient was lying down comfortably. The baseline readings of BP and HR were calculated as the average of 3 readings at 2-min intervals starting upon arrival (with a difference of below 10%). An 18 G-cannula was inserted, and premedication drugs were administered (metoclopramide [10 mg] and ranitidine [50 mg]). Rapid crystalloid co-load infusion (10 mL/kg of lactated Ringer's solution) was commenced. A subarachnoid block was performed in the sitting position. Ten milligrams (2 mL) of heavy bupivacaine, in addition to 25 µg of fentanyl (a total volume of 2.5 mL), were injected in the L3-L4 or L4-L5 interspace using a 25 G-spinal needle. Following the block, success was assessed 5 min after the intrathecal injection using pinprick. The success of the block was confirmed if the sensory block level was at T4. After the subarachnoid block, mothers were placed in the supine position with left-lateral tilt. Fluid administration was continued up to a maximum of 1.5 L. After delivery, an oxytocin bolus (0.5 IU) was administered over 5 s, followed by infusion at a rate of 2.5 IU/h.
Hypotensive episodes (defined as SBP ≤ 80% of the baseline reading [ie, the decline in the baseline SBP by 20%]) were immediately managed by a vasopressor (NE) bolus delivered according to the group randomization. An additional bolus of the same vasopressor (NE) was delivered if the patient did not respond to the first dose within 2 min. Patients were divided into 3 groups according to the received NE bolus dose: 3-µg group, 4-µg group, and 5-µg group (each included 20 patients). Intraoperative bradycardia (HR less than 55 bpm) was managed by atropine bolus (0.5 mg).
In addition to the continuous BP and HR monitoring, umbilical blood gases (PCO2, PO2, and HCO3) at 5 min postdelivery, and Apgar scores of the fetus at 1 and 5 min postdelivery, total vasopressor dosage, incidence of intraoperative bradycardia, nausea, and vomiting were all recorded.
3.6. Study Outcomes
It was hypothesized that increasing the NE dose would successfully treat maternal hypotension with the least side effects regarding the neonatal pH.
The primary outcome was the neonatal concentration of HCO3 from the umbilical cord sample (as a surrogate of neonatal outcome). Secondary outcomes were the rate of the successful management of maternal hypotension (defined as the return of SBP to be > 80% of the baseline reading in the next reading 2 min after administration of the NE bolus), rate of successful management of severe maternal hypotension (defined as SBP ≤ 60% of the baseline reading during the period starting from intrathecal injection of local anesthetic till delivery of the fetus), incidence of reactive hypertension (defined as SBP ≥ 120% from the baseline reading after administration of NE bolus), SBP (baseline reading and the subsequent 12 readings), HR (baseline reading and the subsequent 12 readings), incidence of intraoperative nausea and vomiting, umbilical blood gases (PCO2, PO2, and HCO3) at 5 min postdelivery, Apgar score for the fetus at 1 and 5 min postdelivery, and additional vasopressor requirements.
3.7. Sample Size Calculation
The primary outcome of this study was the HCO3 level of the neonate. In a previous study (6), the mean neonatal HCO3 in preeclamptic patients who received NE was 22.2 ± 1.5 mmol/L. The sample size was calculated to detect a mean difference of 5% (1.11) between the study groups. G Power 3.1.9.2 software was used to calculate the sample size. To have a study power of 80% and an alpha error of 0.05, a total sample size of 57 participants was estimated. This number was increased to 60 participants (20 per group) to compensate for the possible dropouts.
3.8. Statistical Analysis
The data were analyzed using SPSS version 21 (SPSS Inc, Chicago, IL, USA). Categorical data were presented as frequency (%) and were analyzed using the chi-square test. Continuous data were checked for normality using the Shapiro-Wilk test and were presented as mean (SD) or median (interquartile range) as appropriate. Continuous data were analyzed using 1-way analysis of variance (ANOVA) with post-hoc pairwise comparisons using the Tukey or Kruskal-Wallis test according to data normality. Repeated measures were analyzed using ANOVA for repeated measures. The Bonferroni test was used to adjust for multiple comparisons. P values less than 0.05 were considered statistically significant.
4. Results
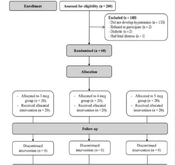
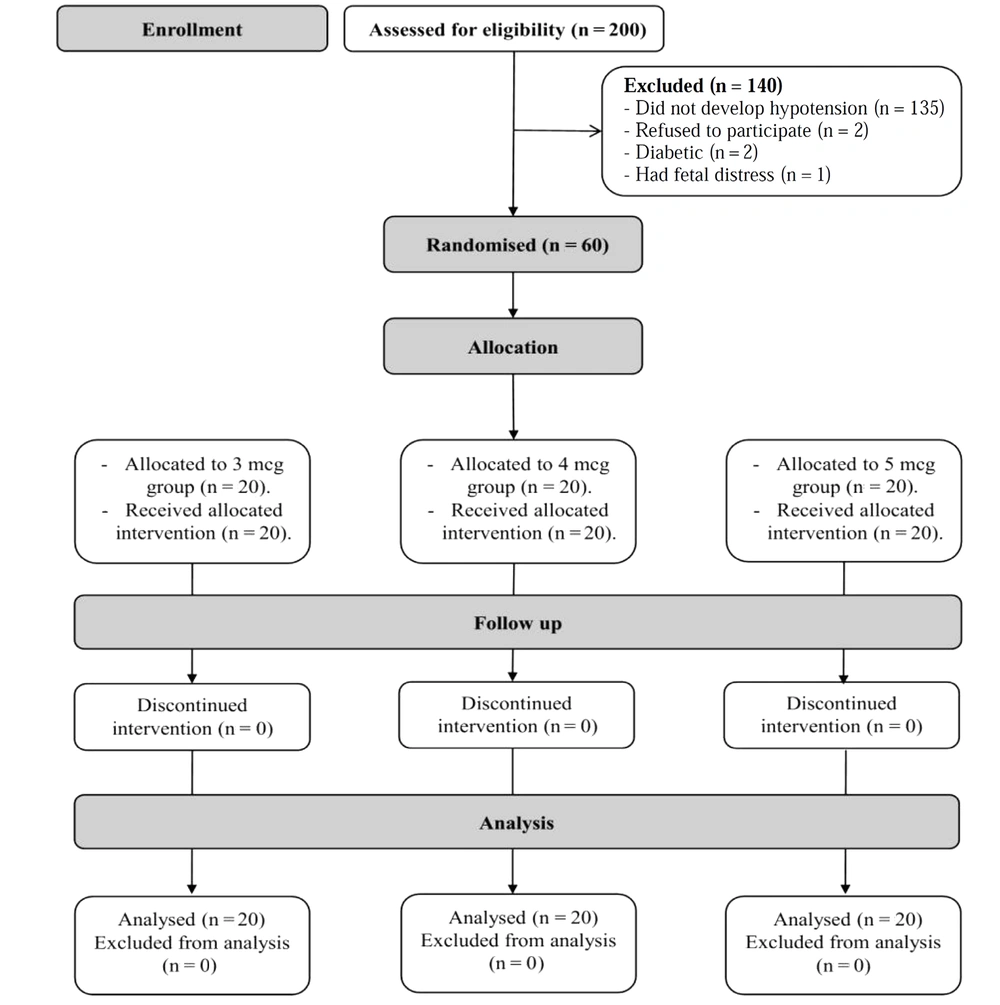
Two hundred mothers with PE were assessed for eligibility. Five mothers were excluded (2 mothers refused to participate, 2 were diabetics, and 1 had fetal distress), and 135 mothers did not develop hypotension. Sixty mothers who developed postspinal hypotension were randomized to the 3 study groups. The 60 mothers were included in the final analysis (Figure 1).
The demographic data and baseline hemodynamic characteristics were comparable in the study groups. The time to delivery was significantly shorter in the 4-µg group than in the 3-µg group (Table 1).
| Variables | (n = 20) | P-Value | ||
|---|---|---|---|---|
| 3-µg Group | 4-µg Group | 5-µg Group | ||
| Age (y) | 28 (4) | 28 (6) | 29 (5) | 0.825 |
| Weight (kg) | 77 (11) | 75 (11) | 77 (10) | 0.822 |
| Height (cm) | 160 (6) | 160 (8) | 160 (6) | 0.956 |
| Baseline SBP (mm Hg) | 153 (8) | 157 (11) | 154 (9) | 0.320 |
| Baseline HR (beat/min) | 93 (8) | 89 (6) | 91 (7) | 0.201 |
| Time till delivery (min) | 20 (18, 28) | 16 (14, 20) b | 18 (15, 20) | 0.026 |
Abbreviations: SBP, systolic blood pressure; HR, heart rate.
a Data are presented as mean (SD), median (quartiles), and frequency (%).
b Significant compared to the 3-µg group.
The Apgar scores at 1 and 5 min postdelivery and the cord blood gases showed no statistically significant differences between the 3 groups (Table 2).
| Variables | (n = 20) | P-Value | ||
|---|---|---|---|---|
| 3-µg Group | 4-µg Group | 5-µg Group | ||
| Apgar score at 1 min | 7 (7, 8) | 7 (6, 8) | 7 (6, 8) | 0.299 |
| Apgar score at 5 min | 9 (8, 9) | 9 (9, 10) | 9 (8, 10) | 0.568 |
| pH | 7.31 (0.06) | 7.33 (0.06) | 7.34 (0.06) | 0.250 |
| PCO2 (mm Hg) | 39 (4) | 42 (4) | 40 (6) | 0.117 |
| PO2 (mm Hg) | 26 (6) | 27 (5) | 27 (6) | 0.815 |
| HCO3 (mmol/L) | 22 (2) | 21 (3) | 21 (3) | 0.944 |
| Lactate (mmol/L) | 2.2 (2, 3) | 2.1 (2, 2.7) | 2.1 (2, 2.9) | 0.999 |
a Data are presented as mean (SD), median (quartiles), and frequency (%).
The mothers received additional doses of the same vasopressor of the study (NE). The total NE doses per mother were calculated and compared between the 3 groups. The number of hypotensive episodes per patient, number of successfully treated episodes per patient, total NE dose per patient, and number of bradycardia episodes per patient were lower in the 4- and 5-µg groups than in the 3-µg group. However, the time to first hypotensive episode, incidence of successfully treated first hypotensive episode, number of mothers who developed bradycardia, and incidence of nausea and vomiting were comparable among the 3 groups (Table 3).
| Variables | (n = 20) | P-Value | ||
|---|---|---|---|---|
| 3-µg Group | 4-µg Group | 5-µg Group | ||
| Time till first hypotensive episode (min) | 2 (2,4) | 2 (2,4) | 2 (2,4) | 0.483 |
| Successfully treated first hypotensive episode (%) | 9 (45) | 9 (45) | 9 (45) | 1 |
| Total norepinephrine dose (µg) per patient | 30 (11) | 19 (8) b | 20 (7) b | < 0.001 |
| Hypotensive episodes per patient | 10 (8, 12) | 5 (3, 7) b | 4 (3, 5) b | < 0.001 |
| Successfully treated hypotensive episodes per patient | 3 (3,4) | 3 (2,3) b | 2 (2,3) b | 0.007 |
| Bradycardia episodes per patient | 3 (1.3,4) | 1.5 (0.3, 2) b | 1 (1, 2) b | 0.004 |
| Incidence of bradycardia (%) | 17 (85) | 15 (75) | 17 (85) | 0.641 |
| Incidence of nausea and vomiting (%) | 10 (50) | 9 (45) | 6 (30) | 0.410 |
a Data are presented as mean (SD), median (quartiles), and frequency (%).
b Significant compared to the 3-µg group
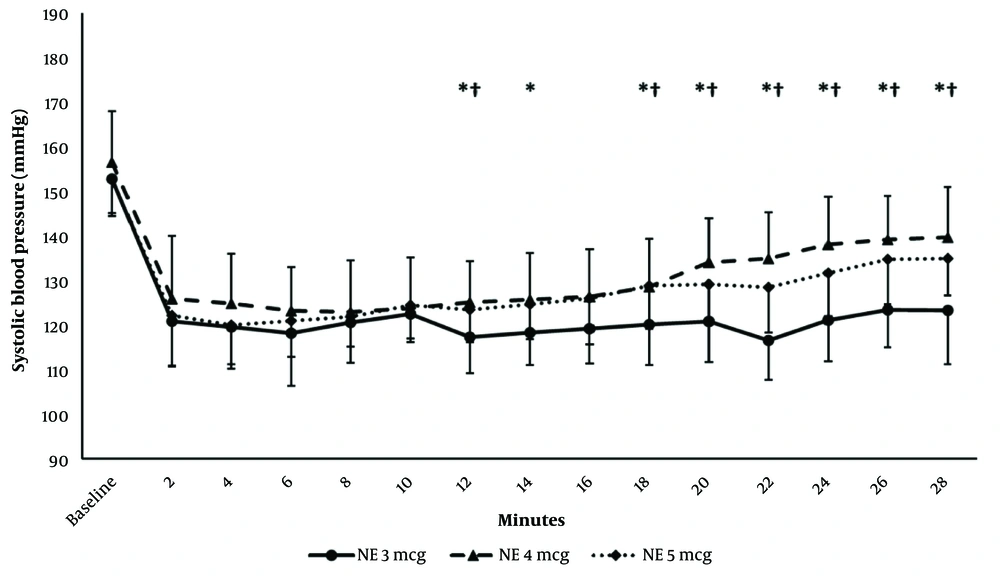
Systolic blood pressure decreased compared to the baseline reading, starting from 2-min postspinal to 28-min postspinal. Systolic blood pressure was generally higher in the 4- and 5-µg groups than in the 3-µg group starting from 12-min postspinal till 28-min postspinal. Systolic blood pressure readings were comparable between the 4- and 5-µg groups (Figure 2).
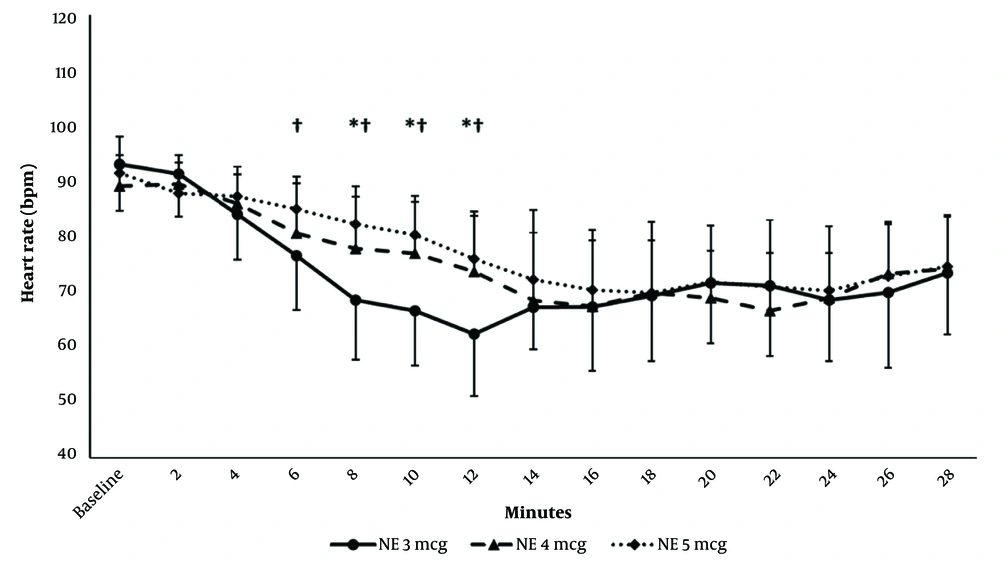
Intraoperative HR monitoring showed that HR decreased compared to the baseline reading starting from 4-, 6- and 2-min postspinal in the 3-, 4-, and 5-µg groups, respectively, till 28 min postspinal. The HR readings were comparable in the 3 groups except at 6-10 min postspinal anesthesia, where it was higher in the 4- and 5-µg groups compared to the 3-µg group (Figure 3).
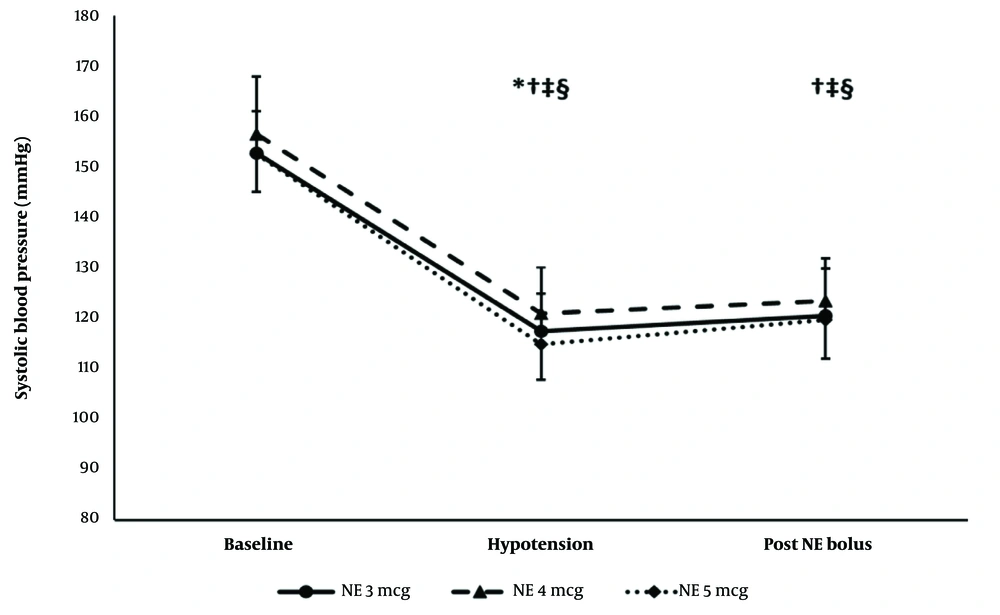
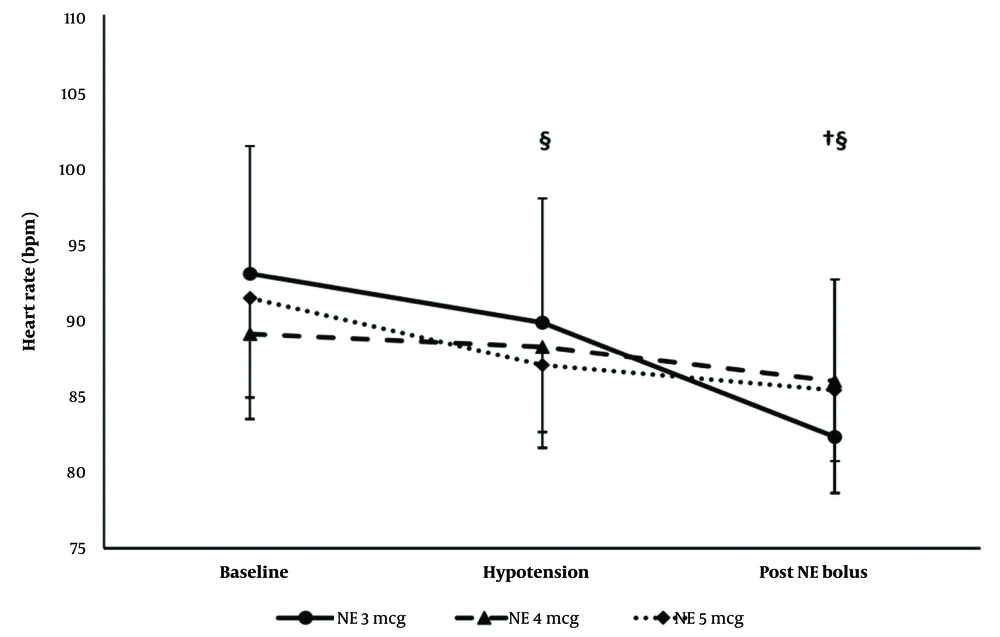
The hemodynamic effects (SBP and HR) of the first NE bolus were comparable in the 3 groups. Systolic blood pressure remained lower than the baseline reading in the 3 groups. The heart rate decreased compared to the baseline reading in the 3- and 5-µg groups only (Figures 4 and 5).
Systolic blood pressure of the first episode. * Significance between the 4- and 5-µg groups. † Significance compared to the baseline reading in the 3-µg group. ‡ Significance compared to the baseline reading in the 4-µg group. § Significance compared to the baseline reading in the 5-µg group
5. Discussion
This randomized, double-blind clinical trial included 60 females with PE who experienced hypotension postspinal anesthesia during cesarean delivery. They were randomly allocated to 3 groups. The results revealed that neonatal outcomes, success in treating the first hypotensive episode, incidence of bradycardia, nausea, and vomiting did not differ significantly between the groups. However, the patients in the 4- and 5-µg groups used significantly lower total NE doses and had significantly fewer hypotensive episodes, fewer successfully treated hypotensive episodes, and fewer bradycardia episodes per patient.
The results of this study shed light on the role of NE in the management of hypotension secondary to spinal anesthesia during cesarean delivery, which is consistent with earlier reports (9, 13, 15). However, the optimum NE bolus dose has not yet been established. Several groups of researchers have been conducting studies for that purpose. Since phenylephrine was the first-line vasopressor used in this setting, Ngan Kee (9) investigated the relative potency of NE compared to phenylephrine in a graded dose-response study. The relative potency of NE and phenylephrine in treating the first hypotensive episode in normotensive women in the study was nearly 13: 1. Another randomized dose-finding study on 100 normotensive patients reported that 100-µg phenylephrine was almost equivalent to 9-µg NE (15). Data regarding NE use in parturient women with PE is even more scarce. Wang et al. (6) conducted a comparative study on a bolus dose of different vasopressors for the treatment of postspinal hypotension in parturients with PE during cesarean delivery. They found that although a bolus dose of NE had comparable efficacy to phenylephrine, it had improved maternal and neonatal safety in those women. Depending on the relative potency ratio reported by Ngan Kee (9), they used an NE dose of 4 µg. Consistent with the current study, 4 µg of NE was effective in managing hypotension. It had comparable efficacy to phenylephrine and ephedrine in that study. Several studies adopted the 4-µg dose when NE was used as bolus either for prophylaxis or treatment of postspinal hypotension. Mohta et al. (16) conducted a randomized trial on patients with PE receiving either phenylephrine or NE (4 µg) for the treatment of postspinal hypotension. Although the number of hypotensive episodes was higher with NE, the number of boluses required for treatment of the first hypotensive episode was significantly higher in the phenylephrine group than in the NE group (indicating the relative potency of NE), resulting in a similar total requirement for the vasopressors in both groups. This again highlights the efficacy of the 4-µg dose. Other studies on normotensive subjects include the study conducted by Puthenveettil et al. (11). Likewise, the 4-µg dose was effective in managing spinal hypotension with a smaller number of boluses compared to phenylephrine. Another randomized, double-blind study adopted a 5-µg dose of NE and compared it with a 100-µg dose of phenylephrine, reporting comparable efficacy (17).
Other studies were concerned with the prophylactic role of NE in preventing the occurrence of postspinal hypotension in normotensive patients. The 6-µg dose was recommended by Onwochei et al. (18) and Sharkey et al. (19) to prevent hypotension. Sharkey et al. (19) stated that given the efficacy of the 6-µg dose, the use of higher doses in this situation might cause undesired hypertension.
The measured neonatal outcomes showed no statistically significant differences between the 3 studied doses. Norepinephrine has been reported to be associated with favorable neonatal outcomes (6, 20). A systematic review studying the neonatal and maternal outcomes of vasopressor drugs managing hypotension during neuraxial anesthesia for cesarean delivery stated that NE was associated with the lowest umbilical arterial PaCO2 values (3). Rai et al. (21), in a randomized controlled trial comparing phenylephrine and NE, used an NE dose higher than the current study doses (7.5 µg). Likewise, umbilical cord blood gas analysis and Apgar scores were comparable between both vasopressor groups. A contradicting report by Mohta et al. (17) revealed that the umbilical artery pH was higher using phenylephrine vs noradrenaline, which was explained by the possible placental transfer of NE. The NE dose used for that study was 5 µg.
In this study, the incidence of bradycardia was 75% in the 4-µg group and 85% in the other 2 groups. The first NE bolus dose caused a significant decrease in HR below the baseline in the 3- and 5-µg groups but not in the 4-µg group. During the operation, bradycardia between the 6th and 10th minute in the 3-µg group was significant, while, HR was comparable between the groups. The incidence of bradycardia in this study was higher compared to many reports where NE administration was associated with a lower incidence of bradycardia (11, 14, 18, 19). Hassabelnaby et al. (22) reported that HR was lower after NE administration of 10-µg bolus compared to 6-µg bolus, without significant bradycardia requiring atropine administration in either dose.
The higher NE-dose groups in the current study (4- and 5-µg groups) had a significantly lower total number of NE bolus doses, fewer number of hypotensive episodes, and no significant tachycardia or adverse neonatal outcomes. This would raise the question of whether higher bolus doses of NE should be implemented. Researchers have conflicting recommendations regarding the use of high NE doses. Some authors concluded that higher doses of NE infusion were more effective in the prevention of hypotension (12, 23). Wei et al. (23) recommend 0.07 µg/kg/min (the highest dose in their study) as the optimum dose. Other research groups were more cautious and stated that as long as there was no significant advantage to the higher dose, there was no need to use it for fear of tachycardia, hypertension, or compromised fetal perfusion (22, 24). One recent study evaluated the use of a bolus NE dose of 16 µg compared to ephedrine to maintain BP during spinal anesthesia in normotensive patients undergoing cesarean section. In addition to the efficacy of this high dose in controlling BP, the NE group maintained a lower HR compared to the ephedrine group (2).
5.1. Study Limitations
The sample size was small, and the uterine arterial flow was not measured, which prevented the direct observation of the effect of vasopressors on utero-placental perfusion.
5.2. Conclusion
This study reveals better results from the 4- and 5-µg doses of NE as indicated by the lower total NE doses required to control hypotension, as well as the lower number of hypotensive and bradycardia episodes compared to the 3-µg dose. Other than that, the neonatal outcomes, the success in treating the first hypotensive episode, incidence of maternal bradycardia, nausea, and vomiting were comparable between the 3 groups.