1. Context
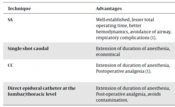
Providing perioperative care for small and/or sick infants, regardless of the type of anesthesia, is certainly a greater challenge for anesthesiologists. Administration of general anesthesia (GA) has a potential risk of airway and respiratory complications in children, especially those with airway diseases (1), besides consuming excessive resources and operating room (OR) time. Spinal anesthesia (SA) is a well-established technique for various surgical procedures in neonates and infants. The addition of the caudal catheter (CC) technique is executed for long and complicated procedures. The combination of these 2 central neuraxial blocks (CNBs, namely, SA and CC techniques) allows us to perform even complex surgical procedures under the sole regional anesthesia technique, thus avoiding GA and its potential complications (1). Various studies are available in the literature that explore the use of CNBs for surgical anesthesia in this population, such as SA as a sole technique (2, 3) or its combination with CC (1). Also, a few review articles have discussed the clinical applications of CNBs in neonates as a part of the broader perspective of pediatric regional anesthesia (4, 5), while only 1 review article (6) analyzed the CNBs exclusively with special emphasis on the safety of adjuvant drugs used. However, to the best of my knowledge, no review article is available in the literature focusing on CNBs as “surgical anesthesia” in this population. In this review article, the individual techniques of CNBs, as well as their possible combinations, are discussed in detail with recent developments and a few notes regarding the “research gap” on this topic. The salient features of the varieties of CNBs with their merits and demerits are described in Table 1.
| Technique | Advantages | Disadvantages | Remarks |
|---|---|---|---|
| SA | Well-established, lesser total operating time, better hemodynamics, avoidance of airway, respiratory complications (1). | Shorter duration of effect. | Continuous SA, addition of various adjuvants need validation. |
| Single-shot caudal | Extension of duration of anesthesia, economical | Infection, impact on the spread of SA, overlapping with SA. | Not studied so far. |
| CC | Extension of duration of anesthesia, postoperative analgesia (1). | Infection, impact on the spread of SA depends on sequence, requires epidurogram. | Well-established based on various studies. |
| Direct epidural catheter at the lumbar/thoracic level | Extension of duration of anesthesia, Post-operative analgesia, avoids contamination. | More possibilities of inadvertent dural puncture. | Not studied so far as a combination with SA. Ultrasound would be helpful to improvise the technique |
Abbreviations: SA, spinal anesthesia; CC, caudal catheter.
2. Search Method
The search conducted on the “PubMed Central" database with the terms “central neuraxial blocks, spinal anesthesia, caudal epidural anesthesia, and infants" as of June 30, 2022, resulted in a total of 76 articles. Only the articles relevant to the topic were included, and a few additional articles were identified as “secondary references" from those initially selected to ensure they align with the topic and fit within the structured headings of this narrative review.
2.1. Spinal Anesthesia
Spinal anesthesia was first administered in 1899 in this population, and subsequently, a case series was published in 1909 (7). Nevertheless, it started gaining widespread application only in the early 1980s when it was considered a better alternative to GA in high-risk newborns/pre-term infants because of its special features such as reduced incidences of postoperative respiratory complications, and apnea, safety in children with difficult airway, susceptible to malignant hyperthermia, etc. (7). It can be used for various infraumbilical and lower limb procedures (7). Varieties of surgical procedures have been successfully accomplished under SA in children, such as lower abdominal surgery (urological procedures and hernia repair), exploratory laparotomy, repair of omphalocele/myelomeningocele, and lower limb orthopedic procedures. The success rate of achieving adequate surgical anesthesia has been observed to be as high as 84% - 95% at experienced pediatric institutions (5). Although SA is a well-established technique in this population, it has still been “under-utilized” in the last 2 decades, as per that review article published in 2021 (5).
Few studies have pointed out the advantages of SA over GA. Spinal anesthesia reduces the incidence of postoperative apnea and respiratory complications compared to GA, even in high-risk infants (8). Spinal anesthesia results in better hemodynamic stability, besides providing less consumption of resources and OR time and less postoperative length of stay when compared to GA (2, 9).
Spinal anesthesia appears safe for infants with congenital heart disease while they undergo non-cardiac surgeries, as the hemodynamic parameters were not different from infants without congenital heart disease (10). Spinal anesthesia does not alter the cerebral oxygen saturation significantly in neonates and infants despite a significant reduction of mean arterial pressure (11). Because of these advantages, SA is certainly a technique of choice, especially for high-risk newborns and infants.
As per the recently published review (5), based on previous studies, the adverse events due to SA were uncommon, with bradycardia occurring in 1.6% and a ‘high spinal’ in 0.6% of infants. Only 3.7% of patients required oxygen supplementation, while oxygen desaturation (pulse oximeter reading of < 90%) happened in only 0.6% of infants. A higher than the intended sensory level was observed in 3.6% of infants who had successful SA, and only a few among these infants required bag-valve-mask ventilation or tracheal intubation.
Complications following SA are infrequent in the pediatric population (5). There were no reports of permanent neurological injuries or fatalities after SA in the pediatric population as per the review published in 2021 (5). On the flip side, the major drawback with SA is the shorter duration of effect. It usually lasts about less than 60 min (1, 5). However, we can extend the duration by adding adjuvants or by providing additional techniques, such as single-shot caudal or CC or an epidural catheter at a higher level. The continuous SA (albeit not commonly practiced), the addition of various adjuvants (3, 5), and their safety in this population (6) need validation from future studies.
2.2. Caudal Catheter Plus Spinal Anesthesia
Caudal epidural is another well-established, easier technique in neonates and infants as a supplement to GA to provide postoperative analgesia. The caudal catheter technique, in combination with SA, has been found to be useful in performing more complex surgeries under sole regional anesthetic techniques (1). By extending the duration of anesthesia, this combination totally avoids the airway intervention, minimizing or completely eliminating the use of opioids, thereby avoiding the potential complications of GA (1). This combination also results in faster restoration of gastrointestinal functions and a lower incidence of abdominal distension and pneumonia when compared to GA in premature/ex-premature/full-term neonates who have undergone elective intestinal procedures (12). Another observational study also concluded that this combination could be considered an effective alternative to GA in high-risk neonates/infants undergoing upper abdominal surgeries (13).
The main drawback of the caudal epidural technique is the concerns of fecal and urinary contamination and infection. A recently published review observed that bacterial colonization was a potential problem despite taking aseptic precautions while inserting a CC. Fortunately, serious complications (namely, meningitis, sepsis, and epidural abscess) are rare (14). Because of concerns about infection and the technical problem of interference of CC fixation with subsequent administration of SA, it is a common practice to execute SA first, followed by the CC technique. Previous studies also followed this method (1, 12, 13). However, this sequence may have an impact on the spread of the intrathecal drug due to postural effects happening during CC insertion.Additionally, the confirmation of CC tip by epidurogram (5, 12) or ultrasound (5), is cumbersome and time-consuming.
2.3. Single-Shot Caudal Plus Spinal Anesthesia
The single-shot caudal can also be combined with SA to extend the duration of anesthesia, although it is not commonly preferred. In this scenario, the question arises which should be given first? If 2 anesthesiologists are available, 1 person can provide the caudal first, followed by SA by the other person. If it is not feasible, SA can be administered either first or after caudal by changing the gloves between the procedures so as to avoid contamination. Regardless of which is first, the single-shot caudal would overlap the SA, resulting in some wastage of duration of anesthesia. Therefore, this combination is not that popular, and only a few anesthesiologists practice it. Nevertheless, this combination is worth investigating in the future as it is economical because it avoids the usage of an “epidural pack," unlike in the CC technique.
The combination of SA and caudal technique (regardless of single-shot or catheter) is a double-edged sword as it has some impact on the spread of the intrathecal drug due to postural effects (happening during caudal if SA is administered first) or more serious concern (ie, infection), if vice-versa.
2.4. Direct Thoracic/Lumbar Epidural Catheter Plus Spinal Anesthesia
The accomplishment of continuous epidural analgesia by placing catheters directly at the thoracic or lumbar region in infants has been in clinical practice since the 1980s (15) and improvised further following the introduction of pediatric epidural needles in the 1990s (16). Few studies found that intermittent epidural top-ups resulted in effective intraoperative and postoperative analgesia, lesser consumption of opioids, and muscle relaxants when combined with GA (15-17).
This technique would eliminate the disadvantages of the CC technique, such as infection, time-consuming and cumbersome confirmation of the tip by portable epidurogram (12), exposure to significant radiation, cost, additional equipment (5), and impact on the spread of SA due to positional changes. It can be safely inserted and fixed, and SA can be administered in a lower space subsequently, like in adult patients.
On the flip side, there is a higher chance of dural puncture with this technique in this population. Because of the anatomical differences in neonates and infants, concern does exist for an increased hazard of neurological injury due to needle trauma in this direct lumbar or thoracic epidural catheter placement (5). Hence, it should be advocated only by experienced anesthesiologists, preferably under ultrasound guidance. Also, we must note the technical nuances, such as changing the angle of the needle, using a micro drop infusion instead of loss of resistance, etc, to improve the success of inserting the catheter in infants (18-20). To the best of my knowledge, an epidural catheter placed directly at the lumbar/thoracic level has not been investigated as a supplement to SA in this population. The direct lumbar/thoracic epidural and the caudal approach have merits and demerits; hence, the debate remains which is ideal in infants (5). Therefore, it is a potential topic for further research, particularly in the background of the recent surge in the application of ultrasound guidance.
2.5. Impact of Central Neuraxial Blocks on Cognitive Function
McCann et al. (21) in their prospective, randomized study recruiting a total of 722 infants concluded that the incidence of hypotension and its treatment requirement were significantly lower in the SA group when compared to the GA group. The final 5-year assessment of neurocognitive outcomes of that study (GAS study) is awaited (21). Despite a lack of definitive data involving human subjects, the US Food and Drug Administration warned that there is a potential impact of GA on neurocognitive functions for children aged < 3 years, more so for procedures lasting > 3 h or with multiple exposures (5). Hence, it is prudent to avoid GA completely by advocating CNBs or reducing the requirements of GA drugs by combining them with CNBs.
2.6. Ultrasound Applications
Recently, ultrasound has become an important tool in all walks of anesthetic practice. Pre-procedural ultrasound examinations have reduced the time to perform the CNBs in children (22). Chawathe et al. (23) performed the first ultrasound-guided visualization of lumbar epidural catheters and could locate them in 10 infants less than 5 months of age. In a landmark study involving 180 infants and children, Kil et al. (24) demonstrated that the pre-procedural ultrasound measurement of skin to the ligamentum flavum distance was highly correlated with the depth of needle insertion. Willschke et al. (25) concluded that ultrasound evaluation of the spinal cord provides valuable information for the placement of epidural catheters in neonates, besides guiding the proper identification of the tip of the needle, as well as the spread of local anesthetic in the epidural space in real-time. Although the caudal block is relatively safe and easy to perform, it can rarely result in dural puncture. Ultrasound guidance helps to produce a higher first puncture success rate with a significantly lower rate of complications, such as intravascular or subcutaneous injection. Also, the placement of the epidural catheter through the caudal route to the desired lumbar or thoracic level could be accomplished accurately with ultrasound guidance. Hence, ultrasound would be preferable for caudal blocks in infants, considering its risk-benefit and cost-effectiveness ratio (26).
2.7. Sedation During Central Neuraxial Blocks
The method of sedating the child just to cooperate (“calm and immobile”) (5) for performing the CNBs varies according to the discretion of the anesthesiologists or the institutional practices. The common methods adopted are oral midazolam (0.5 mg/kg) administered 30 min prior to shifting to OR (1), intravenous dexmedetomidine (1), or just a pacifier dipped in sugar water (7). Intravenous administration of short-acting opioids can also be considered. The requirement of supplementary sedation was 15% - 24% of infants for the accomplishment of SA, with most of them sedated comfortably by using a low-dose dexmedetomidine infusion or intermittent doses of midazolam and/or fentanyl (5). However, it is better to avoid inhalational agents as much as possible, particularly in children who are potential candidates to develop respiratory complications (7).
3. Conclusions
Different combinations of CNBs (such as SA plus single-shot caudal or catheter placed at the caudal/lumbar/thoracic level) are better alternatives than GA in neonates and infants. However, each combination has merits and demerits, thus requiring future studies to assess which is better. Especially an epidural catheter placed at the lumbar/thoracic level as a supplement to SA is a great topic for further research.