1. Background
Postmastectomy pain syndrome (PMPS), a chronic neuropathic pain condition, is a common sequelae of breast cancer surgery, with reported incidence rates ranging from 20% to 70% (1-3). Yoga, as a complementary and alternative medicine (CAM) approach, has been extensively explored as a potential intervention for cancer patients, primarily for psychological health and quality of life improvements (4-6). A literature review revealed two studies evaluating the impact of yoga on pain symptoms in breast cancer surgery patients; however, these studies were limited by small sample sizes, the absence of a control group, the use of non-validated pain scales, and a lack of evaluation for the neuropathic components of pain and PMPS (7, 8).
MicroRNAs (miRs) play a role in gene expression, potentially altering protein expression and contributing to the development of long-term hyperexcitability of nociceptive neurons through peripheral and central sensitization (9). Recently, various miRs have been explored for their roles in acute inflammatory nociception and neuropathic pain, spinal cord regeneration, basic cellular functions and oncogenesis, and the regulation of cardiomyocyte hypertrophy and gastric cancer carcinogenesis (9-15). However, their role in the pathogenesis of chronic persistent postoperative pain remains unexplored.
A search of PubMed and MEDLINE revealed no studies evaluating the modulation of microRNA-133B (miR-133B) levels in PMPS following a yoga intervention. Furthermore, data on the incidence of PMPS in the Indian/Asian population is lacking.
2. Objectives
This study was initiated to investigate the role of miR-133B in the pathogenesis of PMPS following a yoga intervention, along with an integrated approach using thoracic paravertebral block and pregabalin as the primary outcome. Secondary outcomes included the incidence and severity of PMPS, the detailing of neuropathic components of PMPS, and assessments of psychological health and quality of life.
3. Methods
This randomized controlled study was carried out after receiving approval from the Institutional Ethics Committee for Human Research at a tertiary care institute in India and was registered at the Clinical Trials Registry- India (http://ctri.nic.in) under the identifier CTRI/2017/11/010675. Written informed consent was obtained from the participants. The study included patients of ASA physical status I – II, aged between 20 and 65 years, who were undergoing modified radical mastectomy for breast cancer. Patients with a history of seizure disorders, any contraindication to pregabalin, gabapentin, or other opioids, diagnosed diabetes mellitus or renal insufficiency, or those currently enrolled in another yoga program were excluded.
Preoperatively, all patients started on pregabalin 75 mg twice daily, beginning a day before surgery and continuing until four weeks post-operatively. On the third postoperative day, patients were randomly assigned to one of two groups of 20, each using computer-generated random number tables. Both the control and experimental groups received an integrated pain management approach that included pregabalin and thoracic paravertebral block; additionally, the experimental group received yoga as an intervention. Allocation concealment was ensured using sequentially numbered opaque sealed envelopes.
Before surgery, all patients were briefed on the details of various questionnaires and scales. A detailed psychological evaluation was conducted using the Hospital Anxiety and Depression Scale (HADS) during the perioperative period (normal HADS score = 0 - 7, borderline abnormal = 8 - 10, and abnormal ≥ 11). The functional status of the patients was assessed using the Activity Assessment Scale (AAS), a 12-point questionnaire.
A standard anesthesia technique was employed for all patients, consisting of preoperative fluoroscopy-guided thoracic paravertebral catheter insertion, followed by the administration of general anesthesia (GA).
A multimodal approach to perioperative pain management, including intravenous paracetamol and paravertebral analgesia, was maintained for 72 hours. On the third postoperative day, patients in the Yoga group were introduced to the “Anulom-Vilom” breathing exercise through a physical demonstration by a yoga instructor, and understanding was reinforced by displaying and sharing a two-minute video.
3.1. Methodology for Gene Expression
Blood samples for the gene expression study were collected preoperatively and then again on postoperative days 30 and 90. The analysis of miR-133B gene expression in blood was conducted using a CFX Connect Bio-Rad two-color high-resolution melt (HRM) real-time PCR, following these steps:
3.1.1. Step 1: Extraction of RNA from Blood Samples
RNA was isolated from whole blood using Trizol BD (Ambion, Invitrogen) and RNA Clean and Concentrator™ 5 (Zymo, USA), following the manufacturer's protocol.
3.1.2. Step 2: Synthesis of Complementary DNA
The extracted total RNA was used as a template for the synthesis of synthesis of complementary DNA (cDNA). Preferably on the same day, total RNA (1µg) was converted into first-strand cDNA using microScript microRNA cDNA Synthesis (NORGEN BIOTEK CORP, Canada) according to the manufacturer's protocol.
3.1.3. Step 3: Quantification of miR-133B Gene Expression by Real-time PCR
A real-time quantitative polymerase chain reaction (RT-qPCR) experiment was carried out to measure the expression of the miR-133B gene in the blood samples of the subjects. The qPCR reactions were conducted using the CFX Connect Bio-Rad Real-time PCR system.
3.2. Cycling Conditions for the Real-time PCR Assay
3.2.1. For miR-133B
Initial denaturation at 95°C for 3 minutes, denaturation at 95°C for 20 seconds, annealing/extension at 52°C for 30 seconds, for a total of 40 cycles.
3.2.2. For SN U6
Initial denaturation at 95°C for 3 minutes, denaturation at 95°C for 10 seconds, annealing/extension at 52°C for 30 seconds, for a total of 40 cycles.
Gene-specific primer sequences were utilized for amplification. The confirmation of the gene specificity of the primer nucleotides was carried out using the NCBI-BLAST search program.
3.3. Primer Sequences of Genes Used in the Quantitative Real-Time PCR Assay (A Universal Reverse PCR Primer was Provided in the cDNA Kit)
3.3.1. Primer Sequences for miR-133B
Forward: 5'-TTTGGTCCCCTTCAACCAGCTA-3'.
3.3.2. Primer Sequences for SN U6
Forward: 5'-CAAGGATGACACGCAAATTCG-3’.
In this study, RT-qPCR was utilized to perform fold change analysis to evaluate the differential expression of genes. Gene expression normalization was achieved using SN U6 as reference genes, with delta Ct (cycle threshold) determination and calculations based on the equation described by Enquobahrie et al. In RT-qPCR assays, a positive reaction is indicated by the accumulation of a fluorescent signal, with Ct defined as the number of cycles required for the fluorescent signal to exceed the background level. Cycle threshold levels are inversely proportional to the quantity of target nucleic acid in the sample, meaning lower Ct values indicate a higher quantity of target nucleic acid (16).
3.4. Additional Parameters
In the postoperative period, the intensity and quality of pain were thoroughly evaluated using three different questionnaires at specified intervals (end of 24 hours and on days 3, 14, 30, 60, 90, and 120 postoperatively). All patients were instructed on how to respond to various validated questionnaires, including the Visual Analogue Scale (VAS), Pain Detect Questionnaire (PDQ), and Neuropathic Pain Symptom Inventory (NPSI) (17-19). Patients were identified as having PMPS if they experienced chronic pain in the anterior aspect of the thorax, axilla, and/or upper half of the arm, starting after mastectomy and persisting for three months or longer after surgery (3).
In cases of unsatisfactory pain relief, where VAS scores were ≥ 3/10, rescue analgesia (tablet acetaminophen 325 mg + tramadol 37.5 mg) was provided, and records were maintained in a pain diary.
The functional status of the patients was assessed using the AAS, a 12-point questionnaire. Quality of life was evaluated using the short-form 12 (SF-12) questionnaire on the 3rd, 60th, 90th, and 120th days postoperatively. Any adverse side effects were documented.
3.5. Visual Analogue Scale (VAS) Pain Scores
The intensity of pain was meticulously evaluated using the VAS at various intervals, with pain categorized as mild (1 - 3), moderate (4 - 6), and severe (> 7).
3.6. Pain Detect Questionnaire
The neuropathic component of pain was assessed through PDQ scoring at specified intervals, with scores ranging from 0 to 35. Scores from 13 - 18 suggest a possible neuropathic component and scores greater than 18 indicate a high likelihood of neuropathic pain.
3.7. Neuropathic Pain Symptom Inventory
The NPSI was assessed at specified intervals, with scores ranging from 0 to 10.
Quality of life was evaluated using the SF-12 questionnaire, which comprises two components: The physical component summary (PCS) and the mental component summary (MCS). Scores range from 0 to 100, with a mean ± SD of 50 ± 10, indicating that scores above 50 represent above-average health status. The AAS includes a set of 12 questions to evaluate functional activity, with scores ranging from 1 (no difficulty) to 5 (not able to do).
3.8. Yogic Intervention
The yogic breathing exercise “Anulom-Vilom” was initiated on the third postoperative day and continued until day 90, for 10 minutes daily following the recommended methodology. Patients kept a diary recording their daily yoga practice and the need for rescue analgesia, if any.
3.9. Methodology of "Anulom-Vilom"
Patients were advised to sit in a meditative posture, keeping their spine and head erect with their eyes closed. They were instructed to inhale through the left nostril for four seconds and exhale through the right nostril for six seconds, then inhale through the right nostril for four seconds and exhale through the left nostril for six seconds, continuing this cycle for five minutes. The breathing should be slow, steady, and controlled, gradually increasing the duration to 10 minutes.
3.10. Statistical Analysis
The statistical analysis was conducted using SPSS software (version 20). Descriptive statistics facilitated the summarization and calculation of the incidence of PMPS at postoperative day 90. At the end of postoperative day 90, the incidence of PMPS in patients undergoing Modified Radical Mastectomy (MRM) for breast carcinoma was compared with the expression of miR-133B using the Student’s t-test. A P-value ≤ 0.05 was deemed significant for comparing changes at designated time intervals. Pearson’s Correlation tests were employed to explore the relationship between the VAS score and the ΔCt for miR-133B at various time points.
4. Results
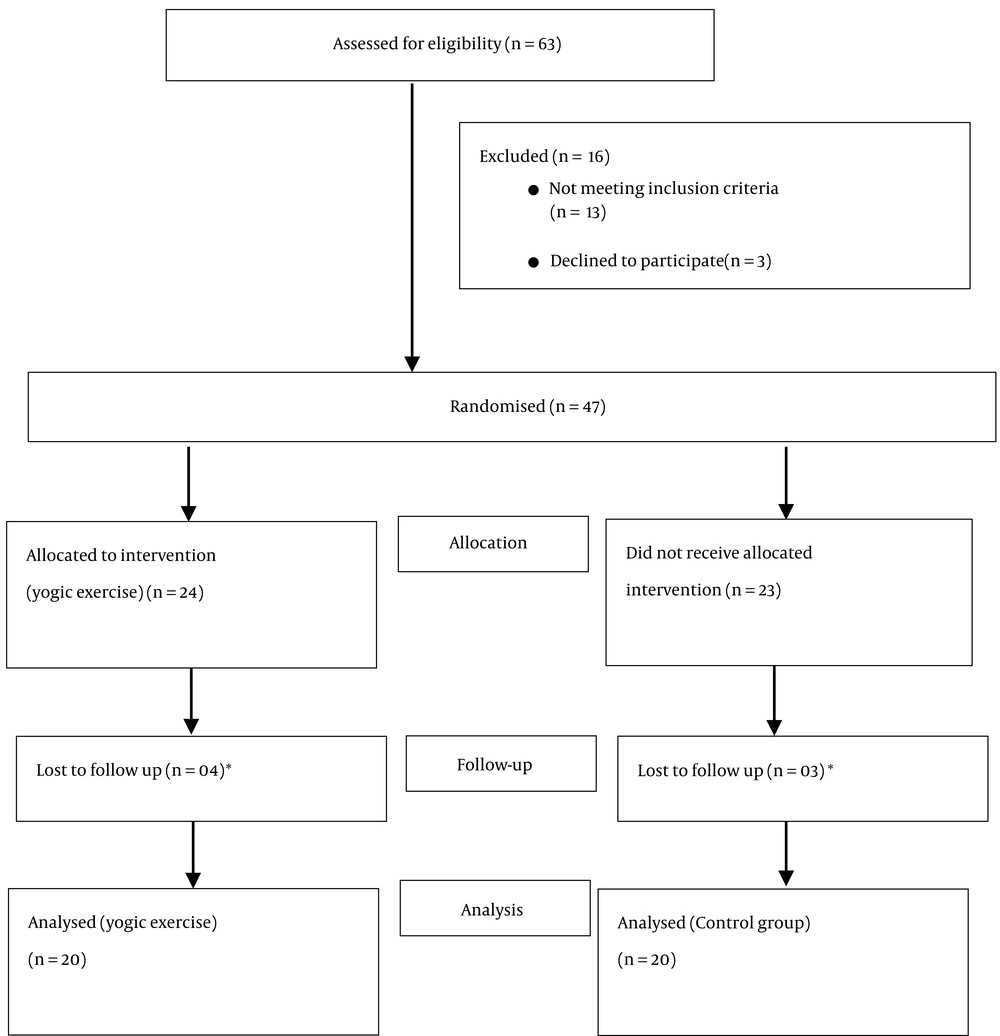
A total of forty-seven patients met the inclusion criteria, of which forty were evaluated as seven patients were lost to follow-up (Figure 1). Demographic parameters were comparable between the two groups (Table 1). A thorough preoperative psychological evaluation of patients was conducted using the HADS and AAS scores (Table 1).
| Patient Characteristics | Control Group | Yoga Group | P-Value b |
|---|---|---|---|
| Demographic parameters | |||
| Age; mean (range) (y) | 49.75 ± 9.89 (35 – 64) | 48.90 ± 10.49 (27 – 64) | 0.79 |
| Weight; mean (range) (kg) | 54.65 ± 4.98 (46 – 67) | 55.75 ± 6.69 (47 – 65) | 0.55 |
| Duration of surgery; mean (range) (min) | 140.50 ± 22.47 (110 – 180) | 143.45 ± 24.96 (110 – 190) | 0.69 |
| ASA Grade- I | 8 (40) | 7 (35) | 0.744 |
| ASA Grade- II | 12 (60) | 13 (65) | 0.50 |
| Preoperative psychologic and activity assessment | |||
| HADS (anxiety score) | 8.10 ± 3.52 | 7.50 ± 3.53 | 0.59 |
| HADS (depression score) | 7.10 ± 3.07 | 6.15 ± 2.45 | 0.28 |
| Pre-operative AAS score | 29.40 ± 11.57 | 23.25 ± 10.56 | 0.12 |
Patients’ Characteristics a
4.1. microRNA-133B Gene Expression
There was a statistically significant (P-value < 0.05) upregulation in miR-133B expression on days 30 and 90 postoperatively when compared to the Control group (Table 2). The Fold Change in miR, 133B expression in the Yoga group (day 30 = 2.265, day 90 = 4.447) was significantly greater compared to the fold change in the control group (day 30 = 1.533, day 90 = 2.399), suggesting a notable increase in miR-133B expression following the yoga intervention, thereby indicating its role in the pathogenesis and modulation of PMPS.
Comparison between ΔCt of miR-133B Gene Expression in Patients with VAS ≥ 3/10 at the End of Postoperative Day 90: An intergroup comparison at day 90 revealed a lower ΔCt of miR-133B, reflecting an upregulation of gene expression in the Yoga group (7.48 ± 1.7 vs. 9.85 ± 2.9), though this difference was not statistically significant (Table 2).
Pearson’s Correlation between VAS Score and miR-133B expression at various time points in both groups: At the end of postoperative days 30 and 90, a significant positive correlation was observed between miR-133B expression and VAS scores in both the control and Yoga groups (Table 2).
| Time Interval | Group C | Group Y | P-Value |
|---|---|---|---|
| Δct of miR-133b at various time points | |||
| Baseline (preoperative) | 10.63 ± 0.693 | 10.19 ± 1.030 | 0.127 b |
| Day 30 | 10.01 ± 1.57 | 9.016 ± 0.616 | 0.011 b |
| Day 90 | 9.36 ± 2.104 | 8.04 ± 1.79 | 0.04 b |
| Comparison between Δct of miR-133b gene expression in patients with VAS ≥ 3/10 at the end of postoperative day 90 | |||
| Baseline | 10.68 ± 0.881 | 10.65 ± 0.33 | 0.965 |
| Day 90 | 9.85 ± 2.994 | 7.48 ± 1.774 | 0.343 |
| Pearson’s Correlation between VAS score and miR-133b expression at various time points in both groups | |||
| Day 30 | 0.910 c | 0.718 c | < 0.001 d |
| Day 90 | 0.904 c | 0.872 c | < 0.001 d |
Δct of miR-133b a
4.2. Other Parameters
The Yoga group exhibited lower mean VAS scores compared to the control group at all measured time points; however, the differences were not statistically significant (Table 3). By the end of the third month, the number of patients in the control and Yoga groups with VAS ≥ 3 were six (30%) and two (10%), respectively (Table 3).
| Time Interval | Control Group | Yoga Group | F-Value (Inter-group) | Inter-group P-Value |
|---|---|---|---|---|
| Visual Analogue Scale (VAS) pain scores | 316.682 | 0.28 c | ||
| 24 hours | 6.50 ± 1.50 | 5.95 ± 1.39 | ||
| Day 3 | 5.15 ± 1.46 | 4.90 ± 1.21 | ||
| Day 14 | 4.45 ± 1.57 | 4.05 ± 0.99 | ||
| Day 30 | 3.70 ± 1.75 | 3.40 ± 0.88 | ||
| Day 60 | 3.00 ± 1.68 | 2.55 ± 0.94 | ||
| Day 90 | 2.35 ± 1.38 | 1.70 ± 1.21 | ||
| Frequency of patients with VAS pain score ≥ 3 at various intervals | ||||
| Day 30 | 14 (70) | 18 (90) | 0.235c | |
| Day 60 | 9 (45) | 7 (35) | 0.746 c | |
| Day 90 | 6 (30) | 2 (10) | 0.235c | |
A more substantial decrease in PDQ scores was noted in the Yoga group, though it was not statistically significant (Table 4).
| Time Interval | Control Group | Yoga Group | Inter-group F Value | Inter-group P-Value |
|---|---|---|---|---|
| 24 Hours | 17.95 ± 6.26 | 15.85 ± 5.38 | 2.109 | 0.155 c |
| Day 3 | 15.5 ± 6.35 | 12.90 ± 4.78 | ||
| Day 14 | 13.0 ± 6.36 | 10.60 ± 5.26 | ||
| Day 30 | 11.20 ± 6.70 | 8.55 ± 4.91 | ||
| Day 60 | 9.80 ± 6.80 | 6.70 ± 4.73 | ||
| Day 90 | 8.20 ± 6.91 | 5.20 ± 5.02 |
4.3. Neuropathic Pain Symptom Inventory
Table 5 shows the NPSI scores for burning sensation, allodynia, and pins and needles. A reduction in NPSI scores was observed in the Yoga group; however, the changes were not statistically significant. Due to the limited sample size, other NPSI Scores (squeezing, pressure, stabbing, electric-shock, and tingling) could not be evaluated due to a lack of sufficient data.
| Time Interval | Control Group | Yoga Group | Inter-group F Value | Inter group P-Value |
|---|---|---|---|---|
| NPSI scores for burning sensation | 0.226 | 0.637 c | ||
| 24 hours | 4.11 ± 3.11 | 4.80 ± 2.72 | ||
| Day 3 | 3.53 ± 2.95 | 3.65 ± 2.41 | ||
| Day 14 | 3.16 ± 2.75 | 2.70 ± 2.17 | ||
| Day 30 | 2.63 ± 2.38 | 1.95 ± 2.01 | ||
| Day 60 | 2.26 ± 2.23 | 1.40 ± 1.78 | ||
| Day 90 | 1.79 ± 1.81 | 0.90 ± 1.48 | ||
| NPSI scores for allodynia | 4.518 | 0.040 c | ||
| 24 Hours | 6.20 ± 2.82 | 4.25 ± 2.97 | ||
| Day 3 | 4.30 ± 2.13 | 2.90 ± 2.19 | ||
| Day 14 | 3.25 ± 1.77 | 2.10 ± 1.80 | ||
| Day 30 | 2.10 ± 1.37 | 1.40 ± 1.46 | ||
| Day 60 | 1.55 ± 1.35 | 0.95 ± 1.09 | ||
| Day 90 | 0.85 ± 0.81 | 0.40 ± 0.68 | ||
| NPSI scores for pins and needle sensation | 0.154 | 0.697 c | ||
| 24 hours | 5.50 ± 3.00 | 5.20 ± 2.87 | ||
| Day 3 | 4.10 ± 2.22 | 3.90 ± 2.33 | ||
| Day 14 | 3.20 ± 1.76 | 3.00 ± 2.02 | ||
| Day 30 | 2.20 ± 1.32 | 2.10 ± 1.44 | ||
| Day 60 | 1.50 ± 1.05 | 1.25 ± 1.07 | ||
| Day 90 | 0.95 ± 0.75 | 0.80 ± 0.76 |
The quality of life, as assessed by the SF-12 questionnaire, indicated an increase in PCS scores at day 90 and an increase in MCS scores at days 60 and 90 in the Yoga group compared to the control group, but these increases were not statistically significant. Similarly, a decrease in AAS scores was observed in the Yoga group at all time points, but the differences were again not statistically significant (Table 6).
| Scale and Time Interval | Control Group | Yoga Group | Inter-group P-Value |
|---|---|---|---|
| SF-12 physical component summary (PCS) | 0.581 b | ||
| Day 30 | 38.70 ± 3.31 | 38.50 ± 3.79 | |
| Day 60 | 44.50 ± 2.68 | 43.80 ± 3.00 | |
| Day 90 | 48.40 ± 2.47 | 49.35 ± 2.18 | |
| SF-12 mental component summary (MCS) | |||
| Day 30 | 40.15 ± 3.16 | 40.65 ± 2.58 | |
| Day 60 | 46.20 ± 2.19 | 47.15 ± 1.95 | |
| Day 90 | 49.55 ± 2.06 | 50.35 ± 1.81 | |
| Activity Assessment Scale (AAS) scores | 0.12 b | ||
| Pre-operative | 29.40 ± 11.57 | 23.25 ± 10.56 | |
| Day 3 | 35.25 ± 16.07 | 28.45 ± 14.799 | |
| Day 60 | 22.85 ± 7.24 | 19.95 ± 6.62 | |
| Day 90 | 15.85 ± 3.46 | 14.25 ± 2.33 |
Short Form-12 Quality of Life Questionnaire (SF-12 QoL) and Activity Assessment Scale (AAS) Scores a
5. Discussion
This is the inaugural human study to evaluate the role of miRNA-133B gene expression in the pathogenesis of PMPS. We observed a significant positive correlation between the ΔCt of miR-133B expression and changes in the VAS score on postoperative day 30 and day 90 in both groups. Additionally, there were decreases in mean VAS scores, PDQ scores, NPSI scores, and improvements in SF-12 scores associated with yoga; however, these were not statistically significant.
MicroRNAs are short, non-coding RNAs essential for normal development in non-muscle tissues and are aberrantly expressed in various cancers (11). When investigating breast carcinoma and the pathogenesis of PMPS with yoga as an intervention, we observed a statistically significant upregulation in miR-133B expression at day 30 and day 90 postoperatively in the experimental group, compared to the control group (P-value < 0.05). This suggests the potential role of yoga as an adjunct to an integrated multimodal pain management approach. Our findings align with a recent study by Novello et al., which demonstrated the downregulation of miR-1 and miR-133B in human osteosarcoma cell lines compared to normal osteoblasts, concluding that the expression of miR-1 and miR-133B may regulate cell proliferation through modulation of MET protein expression (20).
In this study, the yoga intervention combined with an integrated multimodal approach has shown a reduced incidence of PMPS (10%, n = 2) compared to the control group (30%, n = 6); however, this difference was not statistically significant. The reduction in PDQ scores and NPSI scores suggests the potential role of the yoga intervention in alleviating the neuropathic component of pain, albeit not statistically significant except for "Allodynia". Most randomized controlled trials (RCTs) have indicated that yoga-based interventions decrease fatigue, depression, and anxiety and improve other subjective measures of well-being (4, 5, 21-23). This study is the first RCT to assess the role of a yoga intervention alongside an integrated pain management approach in the incidence and severity of a pain modality like PMPS.
Various RCTs on the effects of yoga on breast cancer patients have primarily focused on psychological health and quality of life. A literature search revealed only two articles evaluating the impact of yoga therapy on pain symptoms in patients who underwent breast cancer surgery (7, 8), but these studies faced limitations such as small sample sizes, absence of control groups, the use of non-validated pain scales, lack of immediate postoperative yogic intervention, and no evaluation of the neuropathic component of pain.
In the present study, lower mean VAS scores were observed in the yoga group, though the differences were not statistically significant. A cohort study by Sudarshan et al., involving 14 patients, noted that yoga intervention improved pain symptoms, anxiety, depression, and physical function in breast cancer patients. They utilized the Dallas Pain Questionnaire (DPQ), a 16-item scale to assess the impact of pain on daily activity, work, anxiety/depression, and social life; however, its use in oncology is still limited. Similar to our findings, decreasing trends were noted in the impact of pain on daily activity (6 vs. 13), work and leisure (5 vs. 3), and anxiety/depression (6 vs. 14) compared to the pretreatment mean values (7).
The precise mechanisms by which yoga affects pain pathophysiology remain unclear. Possible mechanisms for yoga's benefits in persistent pain might involve physiological changes that modify the pain experience, such as decreased sympathetic nervous system activity (reflected in reduced heart rate), reductions in inflammatory markers (TNF, interleukin-2, CRP), and stress markers (cortisol), along with increases in flexibility, strength, circulation, and cardiorespiratory capacity (24-29). Yoga may also induce behavioral and psychological changes that influence pain perception (30) by increasing the frequency of positive emotions, potentially reversing the physiological effects of negative emotions, broadening cognitive processes (adopting a broader perspective on problems), and thereby building physical (improved sense of health), social (enhanced social support), and psychological (increased optimism) resources (30-32).
Yoga has been extensively studied in breast cancer patients regarding anxiety, depression, sleep, and quality of life (4-6). In this study, functional status and quality of life were observed to improve with yoga. A systematic review by Cramer et al. assessed yoga's effects on health-related quality of life, mental health, and cancer-related symptoms, finding yoga to be more effective than no therapy (6).
Both groups were comparable in terms of preoperative anxiety, depression, and activity status of the patients. The link between psychological morbidity and the development of PMPS is well documented. Seah et al. recently found that 20% of patients met the criteria for anxiety and depression one year after diagnosis, in contrast to the normal Hospital Anxiety and Depression (HAD) scores in breast cancer patients included in this study (33).
As for adverse events, they were mild and did not necessitate any active intervention. The main limitation of this study is the inability to supervise participants in the Yoga group continuously after their discharge. Nonetheless, patients were encouraged to practice yoga and maintain a diary through phone calls and follow-up visits. Additionally, the study's small sample size and the fact that the data is from a single academic institute may limit its generalizability. Also, the upstream and downstream analysis of miR-133B expression was not performed due to time and cost constraints. Although a strong trend toward improvement in pain intensity and well-being scores was observed, it did not reach a statistically significant level, which could be due to the limited sample size.
5.1. Conclusions
This study is the first to evaluate the expression of miR-133B in PMPS following a yoga intervention in humans. A significant positive correlation was observed between miR-133B expressions and VAS scores at the end of postoperative days 30 and 90 in both groups, with a more significant increase in miR-133B expression noted following yoga. This suggests the potential role of miR-133B gene expression in the pathogenesis of PMPS. Additionally, a decreased incidence of PMPS was observed in the Yoga group (10%) compared to the control group (30%). Improvements in the neuropathic component, functional status, and quality of life were also observed with yoga. This study underscores the importance of integrating yoga with a multimodal pain management approach in patients undergoing breast cancer surgery.