1. Background
Sevoflurane, an inhalant general anesthetic from the ether group, is widely utilized in children due to its rapid induction onset, minimal respiratory tract irritation risk, and low blood solubility, making it considered safe for use. However, prolonged exposure or multiple exposures during the brain's developmental phase are thought to induce neurotoxicity, potentially leading to cognitive impairment (1). Previous studies have proposed several mechanisms for sevoflurane-induced neurotoxicity leading to cognitive impairment, including neuroinflammation (2-4).
Neuroinflammation is crucial in cognitive disorders, characterized by overexpression of proinflammatory factors due to glial activation and immune cell infiltration. Sevoflurane accelerates microglia migration and activation, with activated microglia being the central nervous system's primary cytokine source, releasing proinflammatory cytokines and chemokines (5).
Sevoflurane-induced neuroinflammation involves various mechanisms and signaling pathways, such as NFκB signaling activation through M1 microglia activation, which not only increases M1 polarization but also suppresses M2 activation. Furthermore, sevoflurane-induced NFκB signaling activation can be triggered by increased Ca2+ through the activation of inositol 1,4,5-triphosphate receptors, leading to abnormal calcium release from the endoplasmic reticulum (6). Another pathway involves Tau peptides, where sevoflurane induces Tau migration from neurons to microglia, activating microglia to produce neurotoxic inflammatory molecules and cytokines, such as IL-1β and TNFα, after which Tau can be phagocytosed and secreted extracellularly, resulting in the progressive spread of tauopathy. Repeated sevoflurane exposure also induces tau phosphorylation via GSK-3β, increasing IL-6 production, which causes mitochondrial dysfunction, cell damage, excessive ROS production, and a reduction in hippocampal PSD-95, critical for synaptic plasticity and cognitive function (7, 8).
Propolis is a natural ingredient that has anti-inflammatory and antioxidant effects. Propolis which is commonly called "bee glue", is a product made from bees. Raw propolis usually consists of 50 - 60% resins and balms (including phenolic compounds), 30 - 40% waxes and fatty acids, 5 - 10% essential oils, 5% pollen, and about 5% other substances including amino acids, micronutrients, and vitamins (thiamin, riboflavin, pyridoxine, C, and E). According to literature data, more than 300 compounds including polyphenols, terpenoids, steroids, sugars, amino acids, etc. have been identified in propolis. Flavonoids, polyphenolic compounds, flavones, flavanones, phenolic acids, and their esters are pharmacologically active propolis molecules (9). Previous studies have demonstrated propolis's anti-inflammatory effects (6, 10, 11). Notably, propolis from North Luwu, South Sulawesi, Indonesia, has shown anti-inflammatory properties (12).
2. Objectives
This study aims to explore propolis's potential to prevent memory impairment in a rat model with neuroinflammation due to sevoflurane anesthesia induction during the weaning period. If proven effective, propolis could be developed as a neuroprotective agent in future studies to prevent cognitive disorders resulting from anesthesia.
3. Methods
3.1. Ethical Publication Statement
Ethical approval for this study was granted by the Ethics Committee of Medical Research – Faculty of Medicine, Universitas Indonesia/Cipto Mangunkusumo Hospital (FMUI/RSCM), with the approval number KET-1356/UN2.F1/ETIK/PPM.00.02/2022.
3.2. Animals
We utilized 54 Sprague Dawley rats aged 21 days postnatal (P21) during the weaning period. The study did not focus on sex-dependent effects; therefore, an equal number of female and male rats were not allocated to each group. Instead, rats were randomly assigned to experimental and control groups. The rats were provided by Indonesia’s Agency for Drug and Food Control and were housed in cages in a room with adequate ventilation, a temperature between 18 - 26°C, and a humidity of 30 - 70%. The room lighting was regulated to alternate between light and dark every 12 hours. The rats were divided into a molecular test group, batch 1, were sacrificed at P25 (n = 18), batch 2, were sacrificed at P51 (n = 18), and a memory test group with the Y-maze, batch 3, were sacrificed at P51 (n = 18). Each batch included three groups: Group 1 (control group), group 2 (3% sevoflurane for 2 hours), and group 3 (3% sevoflurane for 2 hours + 200 mg/kg BW propolis every day until decapitation). The Y-maze test for batch 3 was conducted at age P51. Sevoflurane was administered at ages P21, P23, and P25 at a dose of 3% for 2 hours each session. The fresh gas induction flow rate was 3L/minute for the initial 3 minutes (for induction purposes) and then reduced to 1.5L/minute for maintenance.
3.3. Specimen Collection
Rats were euthanized by decapitation at P25 for batch 1 (short period effect) and at P51 for batch 2 (long period effect). The prefrontal cortex was harvested and stored at -80°C.
3.4. Source of Chemicals
Propolis capsule powder was sourced from Tetragonula sapiens bees in the North Luwu district, South Sulawesi, Indonesia kindly gift from Dr. Eng. Muhamad Sahlan, SSi, M.Eng. (Department of Chemical Engineering, Universitas Indonesia). Sevoflurane was purchased from Kalbe Farma, Indonesia.
3.5. Homogenate Tissue
Following the protocol for each group, a rat was euthanized by intraperitoneal injection of xylazine hydrochloride (0.01 mL/kgBW) and ketamine (0.05 mL/kgBW). The prefrontal cortex tissue was then harvested, rinsed with PBS solution to remove excess blood, and stored at -80°C. For homogenate processing, the tissue was first thawed. Then, 100 mg of tissue was weighed, mixed with 1 mL of PBS (pH 7.4), and homogenized at 1500 rpm for 90 seconds. The homogenate was centrifuged at 10 000 G for 5 minutes. The supernatant was collected, and samples could be stored at -20°C.
3.6. Measurement of IL-6, TNFα, IL-10, and PSD-95
Protein levels were measured using ELISA on batches 1 and 2, utilizing prefrontal cortex tissue homogenate in PBS solution. Total protein concentration was determined using the Bradford test before ELISA. The ELISA kits from Bioenzy (Bioenzy, Indonesia, IDN) were used according to the instruction manuals. All protein level measurements used the Sandwich ELISA technique and were expressed in nanogram/mg of total protein.
3.7. Spontaneous Alternation Y-Maze Test
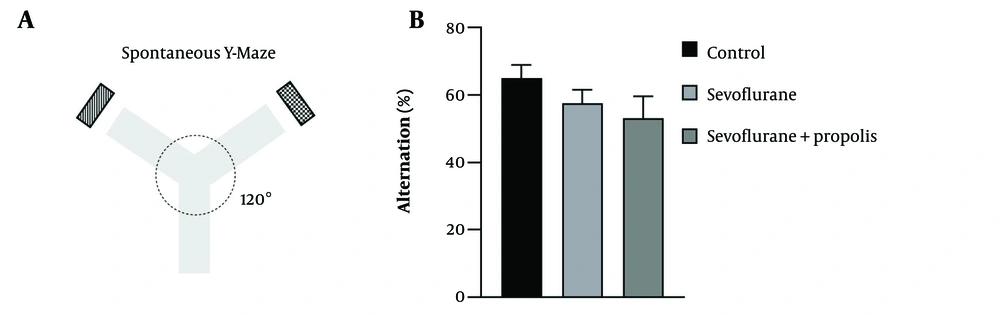
The Y-maze test was conducted to assess spatial working memory and exploratory behavior in rodents, following the protocol by Kraeuter et al. Each arm was set at a 120° angle from the others and labeled with letters A, B, and C to differentiate them. The rat was placed at the center of the maze, allowing it to explore all open arms freely. The number and sequence of arm entries were recorded over ten minutes. Spontaneous alternation reflects the rats' short-term memory, with high alternation indicating excellent spatial working memory. An alternation was counted when the sequence of entered arms was unique (13). The percentage of spontaneous alternation was calculated using the following equation:
3.8. Data Analysis
Statistical analysis was performed using GraphPad Prism version 9 for Windows (ver. 9; La Jolla, CA, USA). The results for protein levels (IL-6, IL-10, TNF-α, and PSD-95) and behavioral tests are presented as mean ± SEM. A normality test with Shapiro-Wilk was conducted before analysis, confirming all data distributions were normal. Protein level results were analyzed using two-way ANOVA, while the behavioral test results were analyzed using One-Way ANOVA to evaluate differences between the experimental groups. Further analysis was done using the Bonferroni post hoc test for multiple comparisons. A P-value < 0.05 was considered statistically significant.
4. Results
4.1. Sevoflurane and Popolis Have No Effect on IL-6, IL-10, TNF-α Levels During Weaning Period
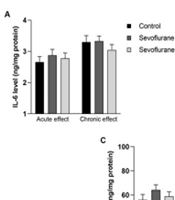
In both the short period effect (P25 decapitation) and long period effect (P51 decapitation) batches, an increase in IL-6 levels was observed in the sevoflurane group compared to the control group, but this increase was not statistically significant. Additionally, there was a decrease in IL-6 levels in the propolis-treated group compared to the sevoflurane-only group, but this decrease was also not statistically significant (Figure 1A).
TNF levels in rats increased in the sevoflurane-only group compared to controls in both groups. There was a slight increase in the sevoflurane+propolis group compared to the sevoflurane-only group in the short period effect batch and a decrease in TNFα levels in the sevoflurane+propolis group compared to the sevoflurane-only group in the long period effect batch, but these changes were not statistically significant (Figure 1B).
IL-10 levels in rats were higher in the sevoflurane-only group compared to controls in both batches, and there were decreased IL-10 levels in the sevoflurane+propolis group compared to the sevoflurane-only group in both batches (Figure 1C).
4.2. Sevoflurane and Propolis Have No Effect on Synaptic Marker PSD-95 During Weaning Period
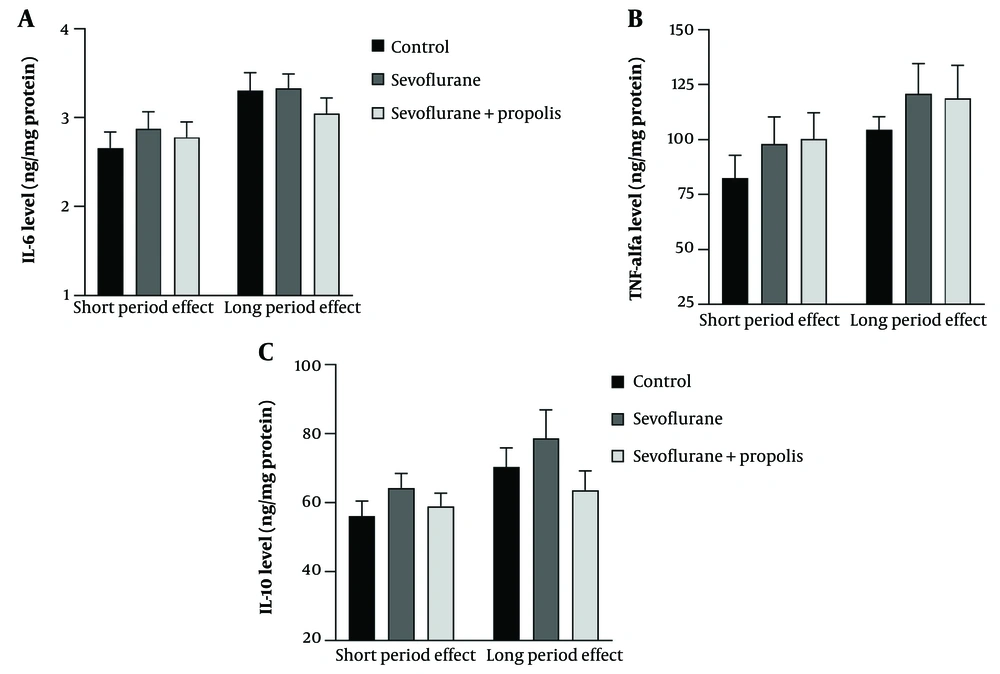
Sevoflurane and propolis had no effect on the synaptic marker PSD-95 during the weaning period. PSD-95 levels in rats increased in the sevoflurane-only group compared to controls in both batches, but this increase was not statistically significant. In the short period effect batch, PSD-95 levels increased in the sevoflurane+propolis group compared to the sevoflurane-only group. In the long period effect batch, there was a decrease in PSD-95 levels in the sevoflurane+propolis group compared to the sevoflurane-only group. There was a statistically significant increase in PSD-95 levels between the control and sevoflurane-only groups from the acute to the chronic batches (Figure 2).
The effect of sevoflurane and propolis on the synaptic marker PSD-95. No treatment effect was observed in either short and long period effect after 3 times repeated exposure of sevoflurane. There is a significant difference between the short and long period effects in the control and sevoflurane groups (** P < 0.01; *** P < 0.001, Two-way ANOVA, n = 6 rats/group).
4.3. Sevoflurane and Propolis Have No Effect on Working Memory Function During Weaning Period
Sevoflurane and propolis had no effect on working memory function during the weaning period. The spontaneous alternation Y-maze test, assessing short-term spatial working memory and executive function through the rats' natural exploring behavior, was conducted for batch 3 (behavior test group) on day 51. Figure 3 shows the percentage of spontaneous alternation on day 51. Propolis did not significantly prevent memory impairment caused by sevoflurane (P > 0.05).
5. Discussion
Our study showed that there were observed increase in the pro-inflammatory factors IL-6 and TNF-α in the group treated with prolonged and repeated sevoflurane compared with the control groups, although this increase was not statistically significant. Interestingly, the IL-10, an anti-inflammation cytokine, is also have a tendency higher in sevoflurane group compare to other groups, either in short effect nor the long period effect, although the increase was not significant. An increase in the anti-inflammatory cytokine IL-10 could inhibit the body's response to inflammatory processes and tissue damage. Altogether, our data showed the effect of prolonged and repeated sevoflurane during weaning period at a dose of 3% for 2 hours administered three times (P21, P23 and P25) only showed a tedency of the increase of proinflamatory marker IL-6 and TNF alfa, and also a tendency of the increase of antiinflamatory marker IL-10, thus it was concluded our model could not produce neurotoxic effects in the rat brain. A significant increase in proinflammatory factors is indicative of neuroinflammation, where neuroinflammation is one of the initial mechanisms for neurotoxicity. This contradicts previous studies where this dose was found to cause neurotoxicity (8). This discrepancy could be due to the different ages of the rat models, with the study by Zhang et al. (8) using mice aged P6, P8, and P10 postnatal, while this study used mice aged 21 days or in the post-weaning period. It is suggested that mice aged 6 days postnatally have brains that are more vulnerable than those of older mice. Another study highlighted that sevoflurane acts like a double-edged sword; on the one hand, it can cause neuroinflammation, but on the other, it has a neuroprotective effect, which also involves the mechanism of NF-κB activation (14).
PSD-95 levels, a protein marker of brain plasticity, experienced a significant increase in the sevoflurane-only group in the long period effect batch compared to the short period batch. This indicates ongoing neurogenesis in the developing brains of rats and suggests that there is no decrease in PSD-95 due to exposure to sevoflurane as the mice age from 25 days to 51 days. This could be attributed to the neuroprotective effects of sevoflurane. A study by Ramos et al. showed a decrease in S100b levels in the serum of pediatric patients after inhaling sevoflurane, compared to serum levels before exposure, where S100b is a marker of glial cell activation and a marker of central nervous system injury (15). Further, the behavior data at P51 days of sevoflurane group also only showed a tendency lower than the control group. This result in line with the result of the proinflammation markers. While the propolis administration did not have any effect on the proinflammatory cytokines nor the memory function.
5.1. Conclusions
Prolonged and repeated exposure to sevoflurane during the weaning period has no effect on IL-6, TNF-α, IL-10, and PSD-95 levels in the prefrontal cortex, in line with working memory function. Additionally, the administration of propolis did not have any beneficial effect on these parameters. Prolonged and repeated exposure to sevoflurane at a 3% dose during the weaning period is insufficient to establish neuroinflammation conditions that would affect synaptic markers and working memory function. Further study is needed to identify a dose of sevoflurane that causes detrimental effects on memory function and to explore the beneficial effects of propolis on this condition.