1. Context
The ancient Egyptians are considered to be the first to discover methanol, but it was first isolated in 1661 by the well-known Irish chemist Robert Boyle (1). Methanol is the primary alcohol with the chemical formula CH3OH. It appears as a colorless, fairly volatile, flammable liquid with a faintly sweet pungent odor similar to that of ethanol. Methanol is the simplest aliphatic alcohol, also known as wood spirit and methyl alcohol (2, 3). Methanol is widely used, particularly in the industry, as an alternative fuel source, solvent, precursor for producing intermediates and synthetic hydrocarbons and polymers, formaldehyde, acetic acid, and so on (4-6). Methanol is a highly toxic compound and is not edible, but small amounts of methanol in the physiological range can be found in the healthy human body due to the fermentation of fruits and vegetables by gut bacteria (3, 7, 8). The range of methanol concentration in the blood is from 0.2 ± 0.035 to 5.37 ± 0.08 mg/L. This small amount is not toxic and does not cause any harm to the human body (9, 10). Unfortunately, there is an increased toxicity with methanol, especially in developing countries and in countries with legal prohibitions on the distribution and sale of alcoholic beverages (11). Methanol intoxication mainly happens because of drinking underground handmade alcoholic beverages (12). Proteomics and genomics techniques, as large-scale analyzers, are high-throughput tools that enable researchers to evaluate diseases at the molecular stage. Due to the lack of comprehensive studies about proteomic and genomic alterations related to methanol poisoning, the current review focuses on the spectrum of metabolic methanol phenomena, including physiological and pathological events, especially changes in serum proteomic profile, and to some extent, genomic changes.
2. Evidence Acquisition
2.1. Search Strategy
The PubMed, Google Scholar, and Scopus databases were reviewed to identify any studies published in English reporting the effect of methanol poisoning on proteomic and genomic patterns in humans. The databases were searched using the keywords "methanol," "intoxication," "poisoning," "proteomic," "genomic," "protein," "gene," "pathology," "metabolism," "genetic alteration," and "protein expression," as well as their combinations. All papers with keywords presented in their titles or abstracts were included in the initial list, while unrelated articles were eliminated. Studies were excluded if they were written in languages other than English, if they had insufficient quality, or if they were duplicated studies. This was done to reflect important advances in proteomic and genomic alterations in methanol poisoning (Figure 1).
2.2. Metabolism and Toxicology of Methanol
Methanol is rapidly absorbed within 30 - 60 minutes depending on the presence or absence of food in the GI tract. It is not a protein-bound compound; rather, it is absorbed directly into the total body water and quickly spreads throughout body tissues. The volume of distribution of methanol has been calculated to be nearly 0.6 - 0.7 L/kg (13-15). The concentration of methanol in the serum peaks after 30 - 90 minutes of consumption (16). The minimal lethal dose of methanol in humans is assumed to be 1 gram per kg of body weight. Ingesting 0.15 mg/kg of pure methanol can lead to poisoning, and death has been reported with 15 - 30 ml of 40% methanol solution (17, 18). Typically, drinking methanol causes acute poisoning rather than chronic poisoning. Signs and symptoms are usually delayed and can appear up to 12 - 24 hours after ingestion (19, 20). The toxic consequences of methanol are caused by the formation of its metabolites. Methanol is metabolized by alcohol dehydrogenase into formaldehyde and then to formic acid, which produces toxic effects in the human body (21). The most frequently detected clinical features of methanol poisoning include central nervous system depression, nonspecific gastrointestinal symptoms, metabolic acidosis, and visual disturbances (22).
2.3. Pathophysiology of Methanol Poisoning
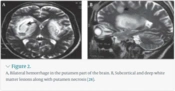
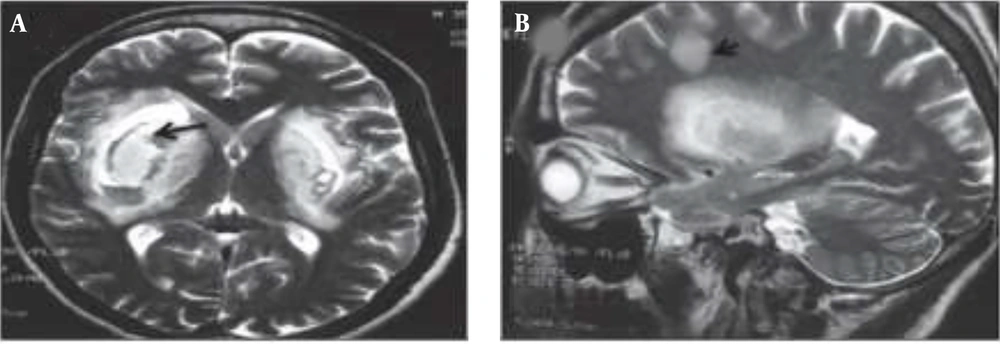
Prognostic and metabolic studies of methanol poisoning (MP) are infrequent (23). The central nervous system (CNS) and the visual system are the key targets of MP (24). Clinical manifestations of MP include gastrointestinal disorders (nausea, vomiting, abdominal pain), CNS suppression, confusion, drowsiness, blurred vision, photophobia, diplopia, early or late blindness, and, less commonly, nystagmus (25). Lesions in the basal ganglia and subcortical white matter, demyelination, and atrophy of the optic nerve arise as a result of MP. Executive dysfunction and memory impairment occur due to lesions in the basal ganglia and disruption of the fronto-striatal circuitry (26). Brain MRI of MP patients has shown sub-acute hemorrhages in the periventricular white matter, putamen, external capsule, and superior cerebellar peduncle on both sides of the brain (Figure 2) (27, 28).
A, bilateral hemorrhage in the putamen part of the brain. B, subcortical and deep white matter lesions along with putamen necrosis (28).
Methanol poisoning can lead to parkinsonism and polyneuropathy, with signs including bradykinesia, hypokinesia, tremor at rest, and slow speech (29-33). Untreated MP can damage parts of the optic system, such as the optic nerve, and pigmented retinal epithelial cells, leading to visual disorders ranging from blurred vision to "snowfield vision" or total blindness in severe poisoning after 72 hours of consumption (34, 35). Optical coherence tomography (CT) reveals nerve fiber swelling due to accumulation of intraretinal fluid in the acute phase, while retinal thickness decreases diffusely in the chronic phase (36). Methanol poisoning interrupts the digestive system by causing changes in tissue mucosa. The esophageal mucosa demonstrates superficial friability, and numerous neutrophils and eosinophils infiltrate the squamous epithelium after focal hemorrhage and capillary congestion. The stomach also indicates brownish mucosa, streak-like erosions, edema, vascular congestion, focal hemorrhage, and discoloration of the pyloric antrum to white (37).
2.4. Proteomics Profile in Methanol Poisoning
Proteomic analysis of biological samples, such as blood serum and urine, is an important research tool in humans for obtaining new data (38, 39). Methanol metabolites induce alterations in proteins by damaging the blood-brain barrier, brain structures, optic nerve, retina, and blood-retinal barrier (40). Human health is threatened by reactive toxins that can damage essential biomolecules such as proteins. Formaldehyde, generated in the human body from methanol, is one of these reactive toxins. Research has shown that formaldehyde, at sufficient levels, poses a serious threat to protein stability (41). Alcohol dehydrogenase (ADH), catalase, and cytochrome P2E1 of the microsomal oxidizing system oxidize methanol to formaldehyde, while aldehyde dehydrogenase converts it to formic acid. Formic acid blocks cytochrome c oxidase in mitochondria, leading to ATP depletion and lactate aggregation by disrupting the mitochondrial respiratory chain (42, 43). Methanol poisoning influences the expression of essential proteins that maintain cell homeostasis. Methanol can react with glutathione and amino acid residues of other proteins, altering their structure and biological activity (40). Methanol intoxication inactivates glutathione-related enzymes in the liver, erythrocytes, and serum, leading to oxidative damage caused by oxidants such as superoxide radicals and phospholipid hydroperoxides. Erythrocytes and other cells continuously produce superoxide radicals and H2O2 at low levels in physiological conditions. Normally, glutathione activity detoxifies H2O2 and superoxide radicals to prevent their reaction with hemoglobin. However, glutathione deactivation by methanol poisoning decomposes hemoglobin into heme and globin, releasing iron (44).
Proteomic analysis of biological samples, such as blood serum and urine, in methanol poisoning reflects impairments of organs. Methanol poisoning causes variable protein modifications such as N-terminal protein acetylation, methionine oxidation, and the formation of the imidazolidone group at the N-terminal valine, as well as N-terminal protein Schiff base formation (45). Intoxicated patients show increased levels of kininogen 1, von Willebrand factor, alpha-2-antiplasmin, plasma serine protease inhibitor, carboxypeptidase N, fibronectin, plasminogen, and coagulation factors, thereby increasing the tendency for coagulation due to the elevation of many coagulation factors (Table 1) (46, 47). Animal studies have revealed that methanol poisoning can affect the immune system's function and induce specific/non-specific immune responses, mainly by increasing oxidative stress and secondary changes in corticosterone levels (48-50). Increased concentrations of lipid metabolism proteins such as apolipoprotein A I, apolipoprotein A II, and adiponectin have been reported (51). Predominantly, the most important proteins related to methanol poisoning are involved in blood coagulation, vitamin A metabolism, and immune response, such as an increase in complement factor I, complement factors C3 and C5 (52, 53). Additionally, lipid transport proteins like apolipoprotein A I, apolipoprotein A II, adiponectin, and retinol-binding protein show increased levels in the blood serum (54, 55).
| Protein Name | Function | Condition | References |
|---|---|---|---|
| Cytochrome c oxidase | Mitochondrial respiratory electron transport | Inhibition | (42, 43) |
| Glutathione reductase | Maintaining the supply of reduced glutathione | Inhibition | (40) |
| Hemoglobin | Transport protein | Degradation | (44) |
| kininogen 1 | Proteinase inhibitor | Up-regulated | (46) |
| Von Willebrand factor | Coagulation factor | Up-regulated | (45) |
| Alpha-2-antiplasmin | Plasmin inhibitor | Up-regulated | (47) |
| Plasminogen | Initiates the fibrinolytic cascade | Up-regulated | (46) |
| Apolipoprotein A I | Lipid metabolism | Up-regulated | (48, 51) |
| Apolipoprotein A II | Lipid metabolism | Up-regulated | (49, 51) |
| Adiponectin | Lipid metabolism | Up-regulated | (50, 51) |
| Complement factor I | Immune response | Up-regulated | (52, 53) |
| Complement factors C3 | Immune response | Up-regulated | (52, 53) |
| Complement factors C5 | Immune response | Up-regulated | (52, 53) |
| Retinol-binding protein | Transporting vitamin A | Up-regulated | (54, 55) |
2.5. Genomic Changes in Methanol Poisoning
Formaldehyde, produced by the enzyme alcohol dehydrogenase (ADH) from methanol, influences the human genome and can increase mRNA transcripts, potentially leading to changes in phenotype due to transcriptional and translational alterations (56, 57). Methanol is not a mutagenic compound, but it has carcinogenic potential, and carcinogenesis can be initiated via reactive oxygen species (ROS) that react with and damage DNA. The guanine base has the lowest oxidation potential among all DNA bases, making it susceptible to damage by ROS. The most common ROS-mediated DNA lesion is 8-Hydroxy-2′-deoxyguanosine or 8-oxo-dG, which is generated by the addition of an oxo group on the C8 position and a hydrogen atom on the N7 of the imidazole ring of dG. If this lesion remains unrepaired, it can cause transversion mutations, where 8-oxoG mismatches with adenine and G:C converts to A:T (58, 59). 8-oxodG also plays a role in the epigenetic regulation of transcription and serves as a driver of the transcription process (60).
A microarray analysis of human blood samples has revealed that methanol can alter the expression of at least 30 mRNA transcripts, most of which are somehow involved in the pathogenesis of Alzheimer's disease (AD). There was also a decreased synthesis of hemoglobin mRNA of HBA and HBB genes (61). Methanol poisoning is a rare event worldwide and mostly occurs in underdeveloped countries and countries with prohibition on alcohol beverages. Therefore, there is a lack of sufficient studies about proteomic and genomic alterations in patients with methanol poisoning. Hence, it is necessary to conduct more studies in this field.
3. Conclusions
Methanol poisoning is associated with adverse effects and mortality; therefore, it should be considered seriously and managed promptly. Delay in treatment could result in permanent damage or even death. Despite limited studies on the proteomic or genomic profile in methanol poisoning, finding biomarkers for the formation of biomarker panels can be valuable tools in managing methanol poisoning. Methanol poisoning alters the levels of proteins involved in blood coagulation, metabolism of vitamins, immune response, and lipid transport. It also indirectly alters gene expression patterns through changes in DNA nucleotides. In summary, biomarker analysis and clinical examination are two important techniques that can lead to the introduction of novel biomarker panels for accurate prognosis, diagnosis, and patient follow-up.