1. Context
Ketamine, a dissociative anesthetic and phencyclidine analog, was originally used as a general anesthetic in the 1960s. Recently, there has been a huge increase in the usage of ketamine in subanesthetic doses for analgesia and other medical indications such as severe depression and schizophrenia (1). Ketamine is a non-competitive open-channel blocker of the N-methyl-D-aspartate receptor (NMDAR) antagonist that functions as a sub-analgesic when the receptor is open. The influx of Na+ and Ca2+ causes depolarization throughout the pain process, enabling the transmission of pain stimuli. Ketamine inhibits the inflow of Na+ and Ca2+ by binding to the NMDA receptor (2-4). It was later discovered that ketamine has effects on several other receptors, including hyperpolarization-activated cyclic nucleotide-gated channels (HCN), GABA uptake and GABA receptors, cholinergic receptors, monoaminergic receptors and transporters, opioid receptors, sigma receptors, voltage-gated sodium channels, and L-type voltage-dependent calcium channels (VDCC) (3, 5). The dosage for ketamine as a sub-analgesic is 0.35 mg/kg given intravenously as a bolus, or 1 mg/kg given continuously every hour. The lowest dosage of 0.15 - 0.25 mg/kg intravenously (i.v.) of ketamine has adequate analgesic properties (5).
Despite the therapeutic effects of ketamine that have been discovered, researchers are still concerned about ketamine's potential toxicity. When administered intravenously at a dosage of less than 0.5 mg/kg for more than 40 minutes, ketamine might result in vestibular disturbances such as nausea, vomiting, and dizziness. Subanesthetic dosages of ketamine can potentially have psychomimetic side effects (5). Long-term use of ketamine for recreational purposes is linked to urological issues, including ulcerative cystitis, hematuria, incontinence, and dysuria (6-8). High doses of ketamine have been observed to induce apoptosis in human urothelial cells, human lymphocytes, brain cells, and PC12 cells through activating mitochondrial apoptotic pathways. It was revealed that at some levels, a shift in [Ca2+] cytosolic might have a detrimental biological impact and result in cell death via an apoptotic process fueled by malfunctioning mitochondria. An increase in [Ca2+] cytosolic was associated with an increase in apoptosis and necrosis.
In vitro investigations on human urothelial cells have shown that ketamine misuse is linked to diseases of the upper and lower urinary system, which were commonly documented in the 1980s. This study demonstrated that ketamine can be lethal at doses over 1 mmol/L, cytostatic at concentrations above 0.3 mmol/L, and able to impede growth at values below 0.3 mmol/L. At non-toxic levels of exposure to ketamine (1 mmol/L), ATP release causes P2Y receptor activation and stimulates calcium release from the endoplasmic reticulum to the cytosol. Ketamine is cytotoxic at exposure levels greater than 1 mmol and causes an uncompensated rise in cytosolic Ca2+ concentrations. Pathological mitochondrial oxygen consumption and ATP depletion are linked to prolonged concentration rises. Due to mitochondrial malfunction, this starts the intrinsic apoptotic process, also referred to as the mitochondrial apoptotic pathway.
Caspases are a unique protease cascade that controls apoptosis. Caspase-8 and Caspase-9 are triggered early in the apoptotic cascade, whereas Caspase-3 is activated near the conclusion. An important caspase in the mitochondrial signaling system is caspase-9, whereas caspase-8 is necessary for apoptosis brought on by death receptors. Caspase-3 activation, which cleaves more downstream effectors, is where the two routes meet. Figure 1 illustrates the apoptotic mechanism. While death receptors trigger the extrinsic route, the mitochondrial pathway is initiated by permeabilizing the mitochondrial outer membrane. A death-inducing signal complex is formed when this route is activated, and this signal complex subsequently causes caspase-8 to become activated, cleaving caspase-3 and activating the general pathway.
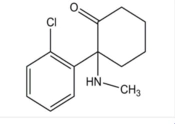
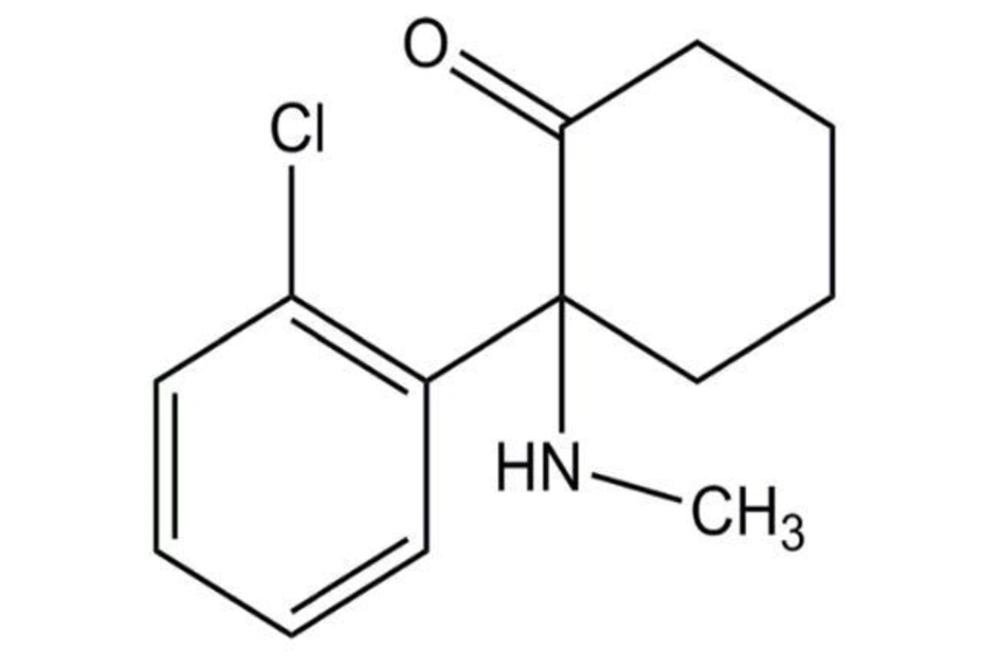
Structure of Ketamine (Source: (4)).
The research examined the cytotoxic mechanism of ketamine in various human cells, including parental cells, neuroblastoma cells, and non-neural Jurkat T-lymphoma cells. They also investigated how ketamine affected cells lacking caspase-9 and caspase-8, two critical cascades in the mitochondrial (intrinsic) process of apoptosis. This study demonstrates that ketamine promotes apoptosis in non-neural and neuronal cells at low millimolar concentrations, but larger doses mostly result in necrosis. Ketamine utilizes the mitochondrial route to induce apoptosis, with no external processes involved. Caspase inhibition can nearly entirely halt ketamine-induced apoptosis, which is concentration- and time-dependent (9).
Additionally, ketamine poisons growing hippocampus neurons in rats. This is assumed to result from ketamine's ability to block calcium oscillations. Transduction begins with a local rise in cytosolic Ca2+ concentration before being distributed throughout the cell in a pattern that is repeated and fleeting. The term "calcium oscillations" refers to the periodic generation of calcium peaks in growing hippocampus neurons via receptors NMDAR. This is presumed to be the mechanism behind the detrimental effects of ketamine on developing rat hippocampus neurons.
1.1. KETAMINE
1.1.1. Discovery
The discovery of ketamine began in the 1950s at Parke-Davis and Company’s laboratories, where Calvin Stevens, a pharmaceutical laboratory researcher at Parke-Davis (the former name of Pfizer), initiated his investigation into the potential of phencyclidine in humans. Initially, chemist Maddox discovered phencyclidine as a candidate for potent anesthesia in animals. However, experiments on monkeys undergoing laparotomy revealed that the animals entered a cataleptic state despite being pain-free. In 1962, Stevens continued Maddox’s work and synthesized a unique series of phencyclidine derivatives in his laboratory (2, 3, 10).
One derivative was selected for human trials as CI-58 [2-(O-chloro-phenyl)-2-methyl-amino cyclohexanone], named ketamine due to the presence of ketone and amine in its compound. In 1965, Corssen and Domino administered ketamine for the first time in humans, and it was introduced into clinical practice by 1970 (10, 11).
1.1.2 Structure
The International Union of Pure and Applied Chemistry has named ketamine as (+/–) 2-(2-chlorophenyl)-2-(methyl-amino)-cyclohexanone. It has a chemical formula of C13H16ClNO with a molecular weight of 237.72. The chiral structure of ketamine consists of two pure optical isomers, named S- and R-ketamine, with each racemate composed of both isomers in equal amounts. The S(+)-isomer is three or four times more potent than the R(-)-isomer as an anesthetic (12).
1.1.3 Mechanism of Action
The binding of NMDA antagonist drugs to NMDA receptors involves various complex processes. There are many compounds known to affect the action of NMDA. These compounds are classified as (1) competitive antagonists; (2) open-channel blockers (e.g., ketamine); (3) non-competitive antagonists; and (4) allosteric potentiators. All of these compounds have different relative potencies at different NMDA receptor subtypes (commonly called GluN1, GluN2A, GluN2B, GluN2C, and GluN2D), so they have different spectra of action. This subtype exhibits a highly heterogeneous distribution in the brain, which may explain the variation in clinical effects caused by different NMDA inhibitory compounds. The GluN2A subtype is found throughout the brain, whereas GluN2B is mainly confined to the limbic system, thalamus, and spinal cord, GluN2C to the thalamus and cerebellum, and GluN2D to the brainstem, diencephalon, and spinal cord (13).
Ketamine's mechanism of action is widely considered one of the most complex in pharmacology due to its interaction with many binding sites, including NMDA receptors, opioid receptors, Na channels, and L-type calcium channels. The main anesthetic effect of ketamine is associated with NMDA receptor blockade using an uncompetitive mechanism with a relatively long knock-off time. N-Methyl-D-Aspartate receptor blockade interferes with normal glutamate excitatory signaling in the central nervous system and is associated with many anesthetic agents (14).
1.1.4. Regulation of Calcium in Neurons
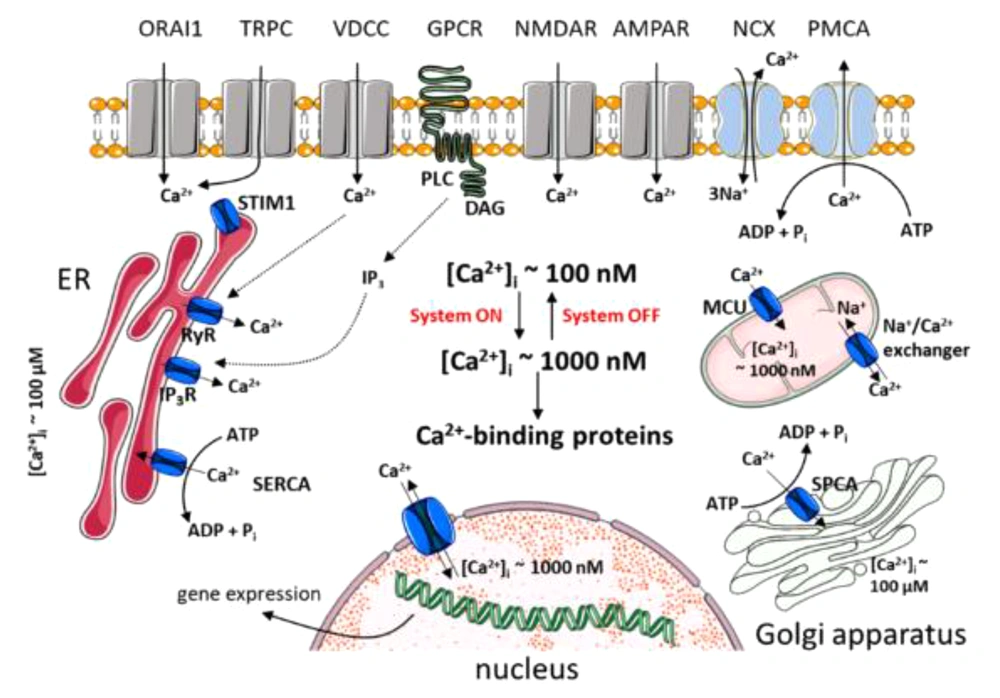
The increase in intracellular calcium concentration (abbreviated as [Ca2+]) can occur in two ways: Extracellular influx and release from intracellular reservoirs such as the endoplasmic reticulum (ER), where the Ca2+ concentration is nearly 1000 times higher than in the cytosol. Several channels can facilitate Ca2+ influx into the cells during depolarization. These include voltage-dependent calcium channels (VDCCs), transient receptor potential channels (TRPCs), calcium release-activated calcium channels (ORAI1), or through glutamate-activated receptor-operated channels: N-methyl-D-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methylisoxazole-4-propionate acid receptors (AMPARs) (Figure 2) (2, 15).
Regulation of calcium in neurons (Source: (2)).
1.1.5. The Role of Endoplasmic Reticulum as a Calcium Reservoir
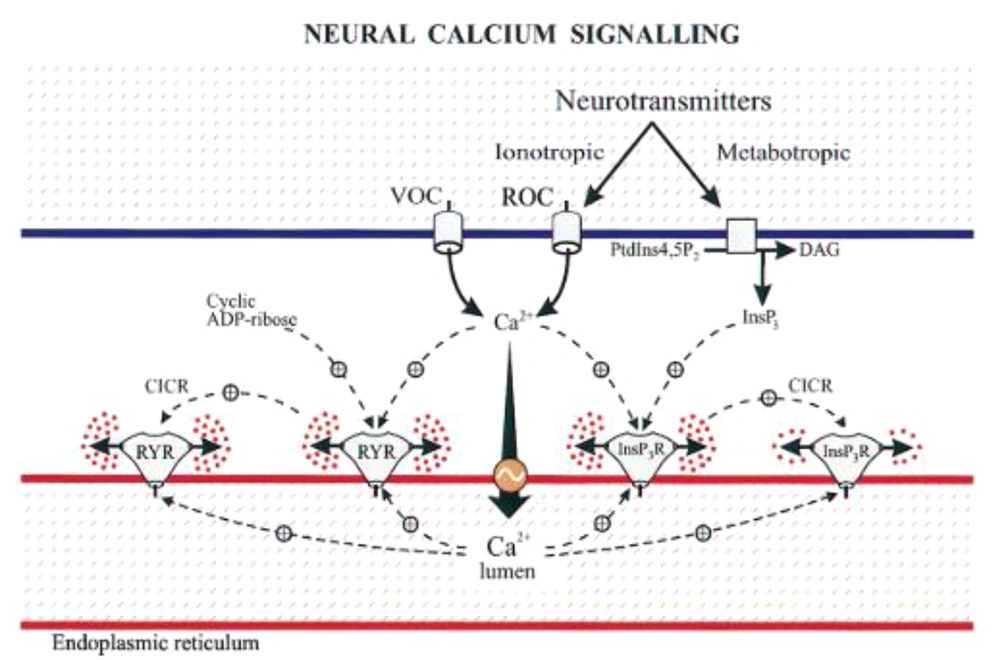
The endoplasmic reticulum is an intracellular reservoir of calcium with a concentration 1000 times higher than in the cytosol. Calcium stored in the endoplasmic reticulum of neurons is an important source of signaling Ca2+ for release upon activation of InsP3R or RyanRs. These two intracellular channels integrate information from the cytoplasm and from within the lumen (Figure 2). The most important property of this organelle is its sensitivity to Ca2+, as both display the phenomenon of Ca2+-induced-Ca2+ release (CICR). This regenerative process amplifies Ca2+ signals from outside and regulates Ca2+ waves (Figure 2). While CICR is usually associated with RYR activity, it is important to emphasize that InsP3R is equally capable of displaying this autocatalytic property. The Ca2+ sensitivity of these two receptors can be affected by various factors, including other messengers (e.g., InsP3 and cyclic ribose ADP) or the degree of loading of stores (Figure 3) (16).
Neural calcium signaling (Source: (16)).
1.1.6. The Interaction of Calcium and Mitochondria
In the previous discussion, we have alluded to the important role of mitochondria in the regulation of intracellular Ca2+. However, apart from mitochondria, other parts of the cell also play a role. Mitochondria-associated membranes (MAM) are important microdomains in Ca2+ homeostasis. MAM is the part that connects the ER and mitochondria, with about 20% of the mitochondrial surface in close contact with the ER. The intimate proximity between the ER and cell membrane creates a microdomain that allows increased mitochondrial calcium concentration ([Ca2+]m) in parallel with cytosolic Ca2+ signals, despite the low affinity for [Ca2+]m uptake (17).
This intimate proximity has been observed between mitochondria and the ER. After the opening of the 1,4,5‑triphosphate (IP3)-gated inositol channels in the ER, the mitochondrial surface is exposed to a higher concentration of Ca2+ than the cytosol, which increases [Ca2+]m. The ER delivers Ca2+ directly to mitochondria via MAM using the InsP3 receptor. The InsP3 receptor is a Ca2+ channel present in the tetrameric ER that functions to release Ca2+ from the ER into the cytosol in response to IP3. Moreover, micro-domains between mitochondria and the cell membrane allow an influx of extracellular calcium through cell membrane channels to mitochondria, providing a link between cellular activation and mitochondrial function. Furthermore, rapid intracellular Ca2+ diffusion avoids organelle overload (17).
1.1.7. Transportation of Calcium to Mitochondria
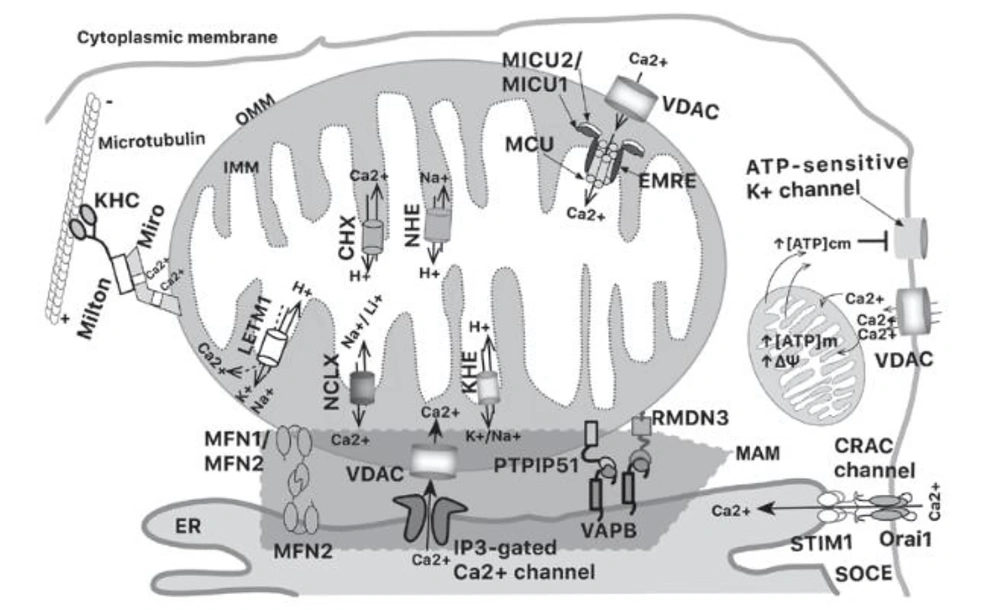
Calcium can be transported into the mitochondria using voltage‑dependent anion channels (VDAC) that exist on the outer mitochondrial membrane (OMM) in excitable cells. Increasing [Ca2+] down to the micromolar range induces higher conductance and maintains open VDAC. However, increases in [Ca2+]m are generally caused by calcium spikes and oscillations, which are usually of short duration (17).
Calcium is carried to the mitochondria by the mitochondrial calcium uniporter (MCU), a ruthenium‑red‑sensitive uniporter that utilizes the negative potential across the inner mitochondrial membrane (IMM). MCU pentamerizes in the IMM as part of a larger molecular weight complex; the other subunits are mitochondrial calcium uptake 1 and 2 (MICU1 and MICU2) and the essential MCU regulator (EMRE). MICU1 and MICU2 function as calcium sensors that allow Ca2+-dependent MCU pore opening. EMRE is a single-pass membrane protein required to allow Ca2+ access. When the concentration of Ca2+ around the MCU is > 1 mM, the channels open and allow Ca2+ to enter the matrix, driven by the large negative membrane potential of the IMM (17).
Meanwhile, the main mechanism underlying the sudden decrease in the amount of calcium in excited cells is the Na+/Ca2+/Li+ exchanger (NCLX); this transporter exchanges mitochondrial calcium matrix for external sodium or lithium. Therefore, to enable Na+/Ca2+ antiporter activity, the Na+ matrix must be exchanged for external H+ by a Na+/H+ exchanger. Additionally, Ca2+ efflux can occur via Ca2+/H+ exchangers, expressed in acidic organelles, including mitochondria. The mitochondrial K+/H+ exchanger (KHE) also participates in mitochondrial calcium dynamics. However, KHE cannot differentiate between K+ and other monovalent cations (Na+ and H+), so it can extrude other monovalents. Another IMM protein with K+/H+ exchange activity is leucine zipper‑EF‑hand containing transmembrane protein 1 (LETM1). LETM1 downregulation can decrease NCLX-mediated Na+/Ca2+ exchange. Additionally, LETM1 is capable of transporting Ca2+ in a pH-dependent manner. When the pH shifts from acidic to basic, LETM1 opens a central cavity that allows the Ca2+/H+ antiporter. Therefore, there is a significant interaction between [Ca2+], [Na+], [Li+], [K+], and [H+] in the mitochondrial matrix and in the intermembrane space, where calcium plays a key role (Figure 4) (17).
Schematic representation of mitochondrial calcium interactions (Source: (17)).
1.1.8. Disturbances in Calcium Regulation Cause Mitochondrial Dysfunction
When the balance between Ca2+ input and efflux is disrupted, there are persistent rises or drops in intracellular Ca2+ concentrations. This alters the usual intracellular Ca2+ pathways and impairs mitochondrial function. Ca2+ influx into mitochondria primarily occurs through VDCCs. Uniporters, which mobilize Ca2+ along the electrochemical gradient due to the negative mitochondrial membrane potential (m), facilitate Ca2+ transport across the mitochondrial inner membrane. The Ca2+-dependent activation of the uniporter and the VDCC is essential for maintaining homeostatic regulation of [Ca2+] (18). Additionally, Ca2+ activates Ca2+-sensitive mitochondrial dehydrogenases in the mitochondrial matrix to promote H+ extrusion, which is crucial for sustaining Ca2+ absorption and ATP synthesis (19).
As a result, a Ca2+ rise that exceeds the capacity of the mitochondria to buffer Ca2+ may decrease the membrane potential and interfere with the electron transport chain, increasing the formation of ROS linked to cell death. Due to the high sensitivity of nerve cells to calcium concentrations, even minor disruptions in Ca2+ homeostasis can have detrimental effects and alter normal brain activity. Furthermore, research has shown that mitochondria are essential organelles for controlling and inducing cell death through specific signaling pathways (20, 21).
1.1.9. Ketamine and Neural Calcium Homeostasis
Recent research has provided evidence that ketamine targets Ca2+-dependent signaling as one of its neural targets. Animal models have been utilized to infer some of ketamine's mechanisms of action, and it has been observed that they align with ketamine's suppression of NMDA function and subsequent downregulation of Ca2+ sequestering proteins. NMDARs on GABAergic interneurons are inhibited, reducing GABA release and causing key excitatory circuits to become overexcited. The disinhibition hypothesis suggests that ketamine-induced NMDAR inhibition leads to significant Ca2+ influx via the glutamate-free channel and mobilization from intracellular reserves, thereby prolonging calcium dynamics and increasing [Ca2+]i in numerous neurons.
The processes driving ketamine-induced [Ca2+]i elevations have not yet been extensively investigated. One theory posits that the brain's inability to maintain Ca2+ homeostasis results from an imbalance in the expression of the "on (influx)" and "off (efflux)" calcium systems. Further investigation into the expression of genes encoding key participants in neuronal Ca2+ handling reveals that ketamine primarily affects the expression of VDCCs and membrane transporters involved in Ca2+ extrusion. In line with this, research by Bustamante et al. on PC12 cells analyzed the effect of ketamine on [Ca2+]i, mitochondrial function, and cell death. This study demonstrated that administration of ketamine at concentrations of 100 µM and 500 µM resulted in various levels of KCl depolarization and inhibited the increase in [Ca2+]i. However, at a dose of 1000 µM, ketamine exhibited a low response to depolarization, indicating interference in managing [Ca2+]i. Additionally, at this concentration, disruption of [Ca2+]i management led to interference with cell survival, ultimately resulting in cell death due to apoptosis mediated by mitochondrial dysfunction. Exposure to high concentrations of ketamine was associated with increased [Ca2+]c levels, leading to elevated apoptosis and necrosis (20).