1. Background
The term "pain," derived from the Greek word "Poine," meaning "penalty," is a complex phenomenon involving both sensory perception and emotional experience. The International Association for the Study of Pain, established by John J. Bonica, a pioneer in pain management, defines pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage (1, 2). Beyond its immediate effects, pain can intricately affect cardiovascular, respiratory, and metabolic pathways, thereby exacerbating patient morbidity. Postoperative pain, in particular, presents a significant challenge as it often hinders patient recovery and necessitates extended hospital stays (3, 4). If left untreated, postoperative pain activates the sympathetic pathway of the autonomic nervous system, leading to the release of catecholamines and subsequent hemodynamic instability. This cascade of events can result in complications such as postoperative ileus and cardiac ischemia, especially in high-risk patients.
In the context of lower abdominal surgeries, regional anesthesia techniques, including epidural and intrathecal anesthesia, are crucial (5, 6). However, intrathecal anesthesia has limitations, such as a short duration of action and an increased risk of post-dural puncture headache. To overcome these limitations, epidural anesthesia serves as a versatile alternative, offering prolonged analgesia, segmental anesthesia, and a lower incidence of post-dural puncture headache (7, 8). Epidural anesthesia allows clinicians to manage chronic pain conditions and maintain hemodynamic stability during surgery, ultimately promoting faster postoperative recovery and reducing hospital stays. Epidural anesthesia often incorporates adjuncts to enhance its efficacy. Among these adjuncts, opioids like morphine have gained prominence due to their ability to bind to mu-opioid receptors in the central and peripheral nervous systems, providing prolonged analgesic effects (9, 10). This makes them a valuable component in postoperative pain management. However, opioids carry the risk of adverse effects such as respiratory depression and pruritus. On the other hand, dexamethasone, a potent glucocorticoid, serves as an alternative adjunct to epidural anesthesia. When combined with bupivacaine, dexamethasone extends the duration of peripheral nerve blockade and analgesia (11, 12). Its anti-inflammatory properties and local vasoconstrictive effects further contribute to enhanced pain relief.
Despite the recognized efficacy of both morphine and dexamethasone as epidural adjuncts, there is a scarcity of comparative studies assessing their analgesic efficacy, time to rescue analgesia, and adverse effects (13, 14). Therefore, this study aims to compare the efficacy of epidural dexamethasone and epidural morphine (EM), both administered with bupivacaine, in patients undergoing total abdominal hysterectomy (TBAH) in a tertiary care setting in Salem.
2. Objectives
This research seeks to bridge the knowledge gap regarding the comparative efficacy and safety profiles of epidural dexamethasone and morphine. The findings will provide valuable insights for optimizing postoperative pain management strategies in clinical practice.
3. Methods
The study included patients aged 30 to 60 years with an American society of anesthesiologists (ASA) physical status of I or II, who were scheduled for elective total abdominal hysterectomy. Exclusion criteria included the presence of an infection at the injection site, a known allergy to local anesthetics, a blood clotting disorder, the use of anticoagulant medication, a history of peptic ulcer disease or diabetes mellitus, a requirement for general anesthesia, a need for an infraumbilical vertical incision, or a refusal to give consent.
Prior to anesthesia administration, patients underwent a series of preoperative investigations to assess baseline health status, including hemoglobin levels, random blood sugar levels, urea and creatinine levels, serum electrolyte levels, chest X-ray, electrocardiogram, blood typing, coagulation profile, and any necessary special serology tests.
During the pre-anesthetic visit, the study protocol was thoroughly explained to all patients, and written informed consent was obtained. Patients fasted for a minimum of 8 hours before surgery, and baseline vital parameters were recorded. In the operating room, patients were connected to monitoring devices, and intravenous (IV) access was established. Local skin infiltration with 2% lignocaine was performed at the T12-L1 intervertebral space, followed by identification of the epidural space using a Tuohy needle. An epidural catheter was inserted, and a test dose was administered to confirm proper catheter placement. Spinal anesthesia was then administered at the L3-L4 space using 0.5% Bupivacaine (heavy).
During closure of the surgical incision, patients received epidural drugs according to their assigned groups: Group D (Dexamethasone) received 2 mL of Dexamethasone 8 mg, while Group M (Morphine) received 2 mL of Morphine 4 mg in normal saline, both mixed with 8 mL of 0.5% Bupivacaine, totaling 10 mL.
Following the procedure, patients were transferred to the post-anesthesia care unit (PACU) and observed for 24 hours. The duration of surgical anesthesia, duration of postoperative analgesia, and any adverse effects were recorded. Pain intensity was assessed using the Visual Analog Scale (VAS), and rescue analgesia was administered as needed.
The independent variables in the study included age, weight, height, ASA physical status, preoperative investigation results, comorbid conditions, duration of surgery, onset, duration, and extent of motor and sensory blockade, and side effects. The outcome variables were the duration of surgical anesthesia, hemodynamic parameters, and side effect comparisons between groups.
Data were entered into Microsoft Excel and analyzed using SPSS version 22. Categorical data were presented as frequencies and proportions and analyzed using the chi-square or Fisher’s exact test. Continuous data were presented as mean and standard deviation, with differences between groups analyzed using the independent t-test. Statistical significance was set at P < 0.05. Graphical representations of the data were generated using MS Excel and MS Word.
4. Results
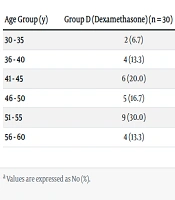
Baseline demographic data, including age, weight, and height, were collected for all participants. However, the specific data is presented in the provided table. The distribution of subjects according to age group is shown in Table 1. A chi-square test of independence revealed no statistically significant difference in age distribution between the two groups (P = 0.639).
| Age Group (y) | Group D (Dexamethasone) (n = 30) | Group M (Morphine) (n = 30) | Total (N = 60) |
|---|---|---|---|
| 30 - 35 | 2 (6.7) | 2 (6.7) | 4 (6.7) |
| 36 - 40 | 4 (13.3) | 3 (10.0) | 7 (11.7) |
| 41 - 45 | 6 (20.0) | 2 (6.7) | 8 (13.3) |
| 46 - 50 | 5 (16.7) | 9 (30.0) | 14 (23.3) |
| 51 - 55 | 9 (30.0) | 9 (30.0) | 18 (30.0) |
| 56 - 60 | 4 (13.3) | 5 (16.7) | 9 (15.0) |
Distribution of Subjects According to Age Group Among Two Groups a
Table 2 analyzes the baseline characteristics of height and weight between the two groups: Group D (Dexamethasone) and Group M (Morphine). The table indicates that the average height for Group D is 157.77 cm with a standard deviation of 5.224 cm, while Group M has an average height of 157.33 cm with a standard deviation of 5.128 cm. The P-value (0.747) is greater than 0.05, signifying no statistically significant difference in height between the groups. This is visually confirmed in the bar chart, where the groups have very similar heights, and the error bars (representing standard deviation) overlap considerably.
Similarly, the table reveals that the average weight for Group D is 57.17 kg with a standard deviation of 5.712 kg, and Group M has an average weight of 56.63 kg with a standard deviation of 5.893 kg. The P-value (0.723) again exceeds 0.05, suggesting no statistically significant difference in weight between the groups. This is also evident in the corresponding weight comparison chart, where the weight values for both groups are very close, and the error bars overlap substantially. Analysis of both height and weight demonstrates that there are no statistically significant differences between Group D and Group M. This suggests that the two groups are comparable in terms of these baseline characteristics, which is crucial for ensuring a fair comparison in any subsequent analyses or comparisons related to the study.
Table 3 and the information provided about Table 4 depict the distribution of subjects according to the American Society of Anesthesiologists (ASA) grade, a scoring system used to assess a patient's preoperative physical status and potential risk of surgical complications, among the two groups: Group D (Dexamethasone) and Group M (Morphine).
| ASA Grade | Group D (Dexamethasone) (n = 30) | Group M (Morphine) (n = 30) | Total (N = 60) | Percentage |
|---|---|---|---|---|
| I | 21 | 20 | 41 | 68.30 |
| II | 9 | 10 | 19 | 31.70 |
| Total | 30 | 30 | 60 | 100 |
Distribution of Subjects According to American Society of Anesthesiologists Grade Among Two Groups
| Parameters | Group D (Dexamethasone) | Group M (Morphine) | P-Value |
|---|---|---|---|
| Duration of Surgical Anesthesia (min) | 135.13 ± 12.057 | 136.20 ± 11.553 | 0.728 |
Comparison of Mean Duration of Surgical Anaesthesia in Minutes Between Two Groups a
The table shows that in Group D, 21 subjects (70.0%) had an ASA grade of I, indicating normal healthy status, while 9 subjects (30.0%) had an ASA grade of II, indicating mild systemic disease. Similarly, in Group M, 20 subjects (66.7%) had an ASA grade of I, and 10 subjects (33.3%) had an ASA grade of II. The P-value of 1.00 indicates no statistically significant difference in the distribution of ASA grades between the two groups. This suggests that both groups have a similar proportion of patients with different levels of preoperative health, ensuring that any subsequent comparisons or analyses are not biased due to baseline differences in health status.
Table 4 summarizes the comparison of the mean duration of surgical anesthesia between the two groups, Group D (Dexamethasone) and Group M (Morphine). The table shows that Group D has an average surgery duration of 135.13 minutes with a standard deviation of 12.057 minutes, while Group M has an average duration of 136.20 minutes with a standard deviation of 11.553 minutes. The P-value of 0.728, which is greater than 0.05, indicates that there is no statistically significant difference in the duration of surgical anesthesia between the two groups. This suggests that the average time spent under anesthesia is comparable between both groups, indicating that surgery duration is unlikely to be a confounding factor in any subsequent analyses or comparisons related to the study.
Table 5 analyzes the changes in heart rate (HR) and mean arterial pressure (MAP) across the two groups: Group D (Dexamethasone) and Group M (Morphine). Heart Rate, the table indicates no statistically significant difference (P-values exceeding 0.05) in heart rate between the groups, both before and after the administration of epidural drugs. This suggests that both groups experienced similar changes in heart rate throughout the surgical process, potentially minimizing the influence of confounding factors related to baseline heart rate variations. Mean arterial pressure (MAP), similarly, the P-values for both intraoperative and postoperative MAP comparisons are above 0.05, indicating no statistically significant differences between the groups. This suggests that both groups exhibited comparable blood pressure responses during and after surgery, potentially minimizing the influence of blood pressure variations as a confounding factor in further analyses.
| Parameters | Time Point | Group D (Dexamethasone) | Group M (Morphine) | P-Value |
|---|---|---|---|---|
| Heart Rate | Before epidural drugs | 83.33 ± 5.115 | 82.83 ± 4.764 | 0.679 |
| Heart Rate | After epidural drugs | 84.07 ± 6.102 | 82.37 ± 5.216 | 0.251 |
| MAP | Intraoperative | 87.73 ± 4.813 | 89.00 ± 7.320 | 0.423 |
| MAP | Postoperative | 87.53 ± 4.883 | 86.93 ± 4.409 | 0.619 |
Comparison Analysis of Heart Rate and Mean Arterial Pressure MAP a
While the average values for heart rate and MAP appear comparable between the groups, it is important to acknowledge that individual responses within each group might still show variations.
Table 6 analyzes the changes in respiratory rate (RR) and oxygen saturation (SPO2) across the two groups: Group D (Dexamethasone) and Group M (Morphine).
| Parameters | Time Point | Group D (Dexamethasone) | Group M (Morphine) | P-Value |
|---|---|---|---|---|
| Respiratory Rate | Intraoperative | 15.77 ± 1.223 | 15.83 ± 1.289 | 0.832 |
| Respiratory Rate | Postoperative | 16.37 ± 1.474 | 16.23 ± 1.357 | 0.717 |
| SPO2 | Intraoperative | 99.50 ± 0.509 | 99.37 ± 0.490 | 0.305 |
| SPO2 | Postoperative | 99.43 ± 0.504 | 99.47 ± 0.507 | 0.799 |
Comparative Analysis of Respiratory Rate and Oxygen Saturation a
respiratory rate (RR), the table demonstrates no statistically significant differences (P-values exceeding 0.05) in respiratory rate between the groups, both during and after surgery. This implies that both groups experienced similar changes in breathing rate throughout the process, potentially minimizing the effect of confounding factors related to baseline respiratory variations.
Oxygen saturation (SPO2), similarly, the P-values for both intraoperative and postoperative SPO2 comparisons are above 0.05, indicating no statistically significant differences between the groups. This suggests that both groups maintained comparable oxygen levels in their blood throughout and after surgery, potentially minimizing the influence of oxygenation variations as a confounding factor in subsequent analyses. While the average values for respiratory rate and oxygen saturation appear similar between the groups, it is important to acknowledge that individual responses within each group might still show variations. Presenting additional information, such as the range of values or the distribution of data across different categories, could provide a more comprehensive understanding of the variability within each group.
Table 7 analyzes the differences in postoperative pain management between the two groups: Group D (Dexamethasone) and Group M (Morphine): Duration of postoperative analgesia, the table shows a statistically significant difference (P-value < 0.001) in the duration of pain medication effectiveness between the two groups. On average, Group D (Dexamethasone) required pain medication for 3.49 hours, whereas Group M (Morphine) needed it for 7.67 hours. This suggests that pain relief lasted longer in Group M (Morphine) compared to Group D (Dexamethasone), indicating that Group M experienced prolonged postoperative pain. Rescue analgesics, the analysis reveals a statistically significant difference (P-value = 0.019) in the requirement for additional pain medication (rescue analgesics) between the groups. In Group D (Dexamethasone), 60% (18 subjects) did not require additional medication, whereas only 13.3% (4 subjects) in Group M (Morphine) did not need it. This further confirms that Group M (Morphine) experienced more pain than Group D (Dexamethasone), as they required more frequent use of rescue analgesics. VAS score, the VAS (Visual Analog Scale) score is a subjective measure of pain intensity. The table shows a statistically significant difference (P-value < 0.001) in the time it took for the VAS score to exceed 4 (indicating moderate pain). The average time for Group D (Dexamethasone) was 3.20 hours, while for Group M (Morphine), it was 8.31 hours. This finding aligns with the previous observations, suggesting that Group M (Morphine) experienced pain for a longer duration and at a higher intensity compared to Group D (Dexamethasone). Conclusion, the analysis indicates that Group M (Morphine) experienced significantly more pain than Group D (Dexamethasone) after surgery. This is evident from the longer duration of postoperative analgesia needed, the higher requirement for rescue analgesics, and the longer time it took for pain scores to improve. These results suggest that epidural dexamethasone may provide more effective and longer-lasting pain relief compared to epidural morphine in the context of postoperative pain management.
| Parameters | Group D (Dexamethasone) | Group M (Morphine) | P-Value |
|---|---|---|---|
| Duration of postoperative analgesia (h) | 3.49 ± 0.51 | 7.67 ± 0.78 | < 0.001 |
| Required rescue analgesics | 0.019 | ||
| No | 18 (60) | 26 (86.7) | |
| Yes | 12 (40) | 4 (13.3) | |
| Duration for VAS score (h) | < 0.001 | ||
| > 4 | 3.20 ± 0.66 | 8.31 ± 0.68 |
Comparative Analysis of Postoperative Pain Management a
Table 8 analyzes the differences in adverse effects and blood sugar levels between the two groups: Group D (Dexamethasone) and Group M (Morphine): Adverse effects, the table shows no statistically significant differences (P-values exceeding 0.05) in the incidence of nausea and vomiting, pruritus, sedation, or respiratory depression between the two groups. While the incidence of these side effects appears higher in Group M (Morphine) compared to Group D (Dexamethasone), the differences are not statistically significant and could be attributed to chance.
| Parameters | Group D (Dexamethasone) (n = 30) | Group M (Morphine) (n = 30) | P-Value |
|---|---|---|---|
| Nausea and vomiting | 2 (6.7) | 7 (23.3) | 0.145 |
| Pruritis | 0 (0.0) | 4 (13.3) | 0.112 |
| Sedation | 0 (0.0) | 2 (6.7) | 0.214 |
| Respiratory depression | 0 (0.0) | 3 (10.0) | 0.176 |
| Blood sugar (8th hour): | 0.001 | ||
| Hyperglycemia | 17 (56.7) | 2 (6.7) | |
| Normal | 13 (43.3) | 28 (93.3) |
Analysis of Adverse Effects and Blood Sugar a
Blood sugar (8th hour), a statistically significant difference (P-value = 0.001) is observed in blood sugar levels at the 8th hour post-surgery. In Group D (Dexamethasone), 56.7% (17 subjects) experienced hyperglycemia (high blood sugar), while only 6.7% (2 subjects) in Group M (Morphine) had high blood sugar. Conversely, 93.3% (28 subjects) in Group M had normal blood sugar levels, compared to only 43.3% (13 subjects) in Group D. This indicates that Group D (Dexamethasone) had a significantly higher incidence of hyperglycemia at the 8th hour after surgery compared to Group M (Morphine). The analysis reveals no statistically significant differences between the two groups regarding common postoperative side effects, such as nausea, vomiting, pruritus, sedation, and respiratory depression. However, a significant difference is observed in blood sugar levels, with Group D (Dexamethasone) experiencing a considerably higher incidence of hyperglycemia at the 8th hour post-surgery. This suggests that while both drugs have comparable safety profiles in terms of common side effects, the use of dexamethasone may be associated with an increased risk of postoperative hyperglycemia.
5. Discussion
Pain, while serving a vital protective function, often becomes intolerable and causes significant distress to patients. Consequently, effective management of pain is crucial for ensuring optimal recovery after surgery (15, 16). Epidural anesthesia has proven to be a valuable tool for providing pain relief following lower abdominal surgeries. However, the ongoing search for an ideal analgesic additive continues, with the goal of achieving a quicker onset of action, effective pain control, and minimal side effects (17, 18). This analysis compared various characteristics and outcomes between two groups: Group D (Dexamethasone) and Group M (Morphine). By analyzing the combined tables and information from the corresponding data, we can gain deeper insights into the findings.
5.1. Baseline Characteristics and Surgical Outcomes
Both groups exhibited comparable characteristics at baseline, including age, weight, height, and ASA grade, which categorizes patients' preoperative health status. Additionally, the duration of anesthesia did not differ significantly between the groups. These observations help minimize the influence of confounding factors in subsequent analyses and comparisons (19, 20).
5.2. Physiological Parameters
Throughout the surgical process, both groups displayed similar physiological responses, as evidenced by no statistically significant differences in heart rate, mean arterial pressure, respiratory rate, and oxygen saturation, both before and after surgery (21).
5.3. Pain Management
The analysis revealed superior pain management in Group D (Dexamethasone) compared to Group M (Morphine). This conclusion is supported by several observations: Group D (Dexamethasone) required pain medication for a shorter duration after surgery. Patients in Group D (Dexamethasone) needed less frequent administration of additional pain medication (rescue analgesics). Group D (Dexamethasone) experienced a faster improvement in pain scores, as measured by the VAS score.
5.4. Adverse Effects
There were no statistically significant differences between the groups regarding common postoperative side effects such as nausea, vomiting, pruritus, sedation, and respiratory depression (22, 23).
5.5. Blood Sugar (8th hour)
An interesting finding emerged regarding blood sugar levels at the 8th hour post-surgery. Group D (Dexamethasone) exhibited a significantly lower incidence of hyperglycemia (high blood sugar) compared to Group M (Morphine). This observation warrants further investigation to understand the underlying causes and potential clinical implications (24, 25).
5.6. Comparison with Previous Research
A meta-analysis conducted by Block et al. reinforced the benefits of epidural analgesia for pain control, regardless of the specific analgesic agent, catheter placement, or timing of pain assessment (26-28). However, when comparing the use of epidural dexamethasone and epidural morphine for pain management, there is no definitive consensus regarding the quality, duration, and side effects of each approach (28-30).
Focus and Mechanism of Action in the Present Study: This randomized controlled trial was specifically designed to compare the effectiveness of epidural dexamethasone and epidural morphine, both administered with bupivacaine, in patients undergoing total abdominal hysterectomy (31, 32). The study's objectives were to evaluate the efficacy of pain relief, the duration until the need for additional analgesia, and any side effects in a tertiary care setting.
Dexamethasone, known for its anti-inflammatory properties, is thought to provide faster pain relief and longer-lasting analgesia compared to morphine, which primarily acts on opioid receptors in the spinal cord.
5.7. Evidence from Prior Studies Supporting the Effectiveness
Numerous studies have confirmed the effectiveness of dexamethasone in managing postoperative pain. Research conducted by Hefni et al. (28), Jebaraj et al. (31), and Waldron et al (32). demonstrated decreased pain scores and reduced opioid use in patients administered dexamethasone compared to those who were not. Additionally, studies by Hjortso et al. and Torda and Pybus highlighted the effectiveness of epidural morphine in controlling postoperative pain (11, 33).
Limitations and Directions for Future Research, the study's smaller sample size, due to time constraints, might limit the generalizability of the findings to the broader population. However, the randomized controlled design ensures internal validity, meaning the results are valid within the study context. The study employed commonly used dosages for both morphine and dexamethasone; exploring the use of higher doses could be beneficial to investigate potential effects on prolonged analgesia and side effects. Additionally, the study did not include long-term follow-up, which limits the understanding of long-term patient outcomes. Future larger, multicenter trials could provide more robust evidence and better inform clinical practice recommendations.
5.8. Conclusions
The study found that patients in both the morphine and dexamethasone groups were similar in baseline characteristics and vital signs. However, those administered morphine experienced a significantly longer duration of postoperative analgesia and took more time for their mean VAS score to exceed 4, indicating enhanced pain management. In contrast, the dexamethasone group required more rescue analgesia and exhibited higher rates of hyperglycemia. There was no significant difference between the two groups concerning nausea and vomiting. These findings suggest a need for further research with larger sample sizes to better understand the trade-offs between the efficacy and side effects of these drugs.