1. Context
Patients admitted to the intensive care unit (ICU) often experience single or multiple organ failure or are at high risk of developing such complications (1). Despite ongoing debate surrounding fluid administration in the management of critical illness, it remains a fundamental and routine component of ICU care (2, 3). Fluid management in critically ill patients is typically structured into three phases: Resuscitation, replacement, and maintenance — each aimed at restoring tissue perfusion, sustaining organ function, and meeting daily fluid requirements (4).
Managing fluids in the ICU is a nuanced and complex task, as both inadequate and excessive fluid administration can adversely affect patient outcomes (4). Many critically ill patients exhibit only a transient response to fluid therapy, partly due to the rapid redistribution of fluids from the vascular compartment and the short half-life of intravascular volume expansion (3). Consequently, fluid overload (FO) is a common occurrence in ICU settings.
Currently, no universally accepted threshold exists for determining the optimal volume of fluid to administer in critically ill patients. This lack of consensus contributes to clinical uncertainty and increases the risk of over-administration (4). As such, fluids should be considered pharmacologic agents — therapeutic in appropriate doses, but potentially harmful when misused (5).
Emerging evidence increasingly suggests that a positive fluid balance, particularly FO, is associated with adverse clinical outcomes in critically ill patients (6). Given the substantial number of studies published in recent years, and the absence of a comprehensive updated synthesis, this meta-analysis aims to clarify and strengthen the understanding of the relationship between FO [(or positive cumulative fluid balance )CFB)] and mortality in critically ill adult populations. By analyzing observational studies from the past decade, we seek to provide a more current and evidence-based perspective on this critical aspect of ICU care.
2. Data Sources
This study adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Figure 1), and the meta-analysis protocol was prospectively registered in PROSPERO (registration number: CRD42023450240).
2.1. Search Strategy
Two independent investigators (APL, EG) systematically searched the PubMed, ScienceDirect, Google Scholar, and EuroPMC databases using the keywords ("critically ill" OR "critical care") AND ("intensive care unit") AND ("positive fluid balance" OR "fluid overload") AND ("mortality") from 2012 to September 2, 2023. Furthermore, references from pertinent papers and reviews were assessed through manual searching. Two independent authors separately removed duplicates and screened the titles/abstracts of the publications from the records. The full texts of articles that potentially met the eligibility criteria were evaluated based on the inclusion and exclusion criteria.
2.2. Eligibility Criteria
The current meta-analysis included all observational cohort studies involving adult critically ill patients (aged ≥ 18 years) receiving care in the ICU who were assessed for fluid excess, defined as positive FO or fluid balance (either daily or cumulative), and that reported mortality risk estimates adjusted for various confounding factors. We excluded the following studies: (1) Cross-sectional, case-control, review articles, preprints, commentaries, editorials, case reports/series, meta-analyses, and conference abstracts; (2) studies in languages other than English.
2.3. Exposure and Outcomes
The terms positive CFB and FO should be distinguished. Positive CFB reflects net fluid balance over time (intake minus losses), while FO refers to fluid accumulation in tissues. A positive CFB does not always indicate FO (7). Many studies used CFB due to the lack of admission body weight data, though FO is more accurately defined as a percentage of body weight gain, commonly using 5% or 10% thresholds. Since no consensus exists on a clinically significant cutoff, FO adjusted for body weight remains the most reliable indicator (8). This analysis also included daily mean balance, based on fluid input and output excluding insensible losses.
The primary outcome was all-cause mortality, categorized as short-term (≤ 30 days), long-term (≤ 90 days), and hospital mortality (any time during hospitalization). We evaluated the impact of FO or positive CFB within the first 24 or 72 hours in the ICU on these outcomes. Subgroup analyses were conducted for general ICU patients and those with acute kidney injury (AKI), sepsis/septic shock, and post-surgical conditions.
2.4. Data Extraction and Quality Assessment
The first author’s last name; country of study; study design; year of publication; percentage of male participants; number of samples; type of study; fluid excess assessment approach; outcome evaluation; comorbid data; adjusted effect estimates with 95% confidence intervals (CI); and confounders were extracted independently by the two authors. Two writers independently evaluated the risk of bias using the Newcastle-Ottawa Quality Assessment for Cohort Studies (9). Studies with scores of 7 were considered of good quality. Discrepancies during the assessment were resolved through discussion.
2.5. Statistical Analysis
Quantitative analysis was performed using STATA version 16.1. Pooled adjusted risk ratios (RR) with 95% CI were calculated using a random-effects DerSimonian-Laird model. Statistical significance was set at P < 0.05. Heterogeneity was assessed using Cochran’s Q test (P < 0.10) and the I2 statistic (>50% indicating heterogeneity). Sensitivity analysis was performed using a leave-one-out approach. Subgroup analyses and meta-regression were conducted based on patient type and fluid excess assessment to explore sources of heterogeneity. Publication bias was evaluated using funnel plots, Egger’s and Begg’s tests, and addressed with trim-and-fill analysis using the linear L0 estimator.
3. Results
3.1. Baseline Characteristics
A total of 34 observational studies (27 retrospective and 7 prospective), published between 2013 and 2023, with a combined sample of 49,467 patients, were included in this meta-analysis (10-44) (Appendix 2 in Supplementary File). Quality assessments are listed in Appendix 3 in Supplementary File. Among the included studies, 19 assessed the association between positive CFB (11, 13, 15-17,19, 22, 23,26-30, 32, 33, 36, 37,39, 40) 6 studies assessed FO (12, 23, 25, 34, 35, 42), 7 studies examined CFB on a per-litre basis (10, 18, 21, 24),(31, 38, 41), and 2 studies evaluated daily fluid balance (14, 40).
3.2. 30-day Mortality
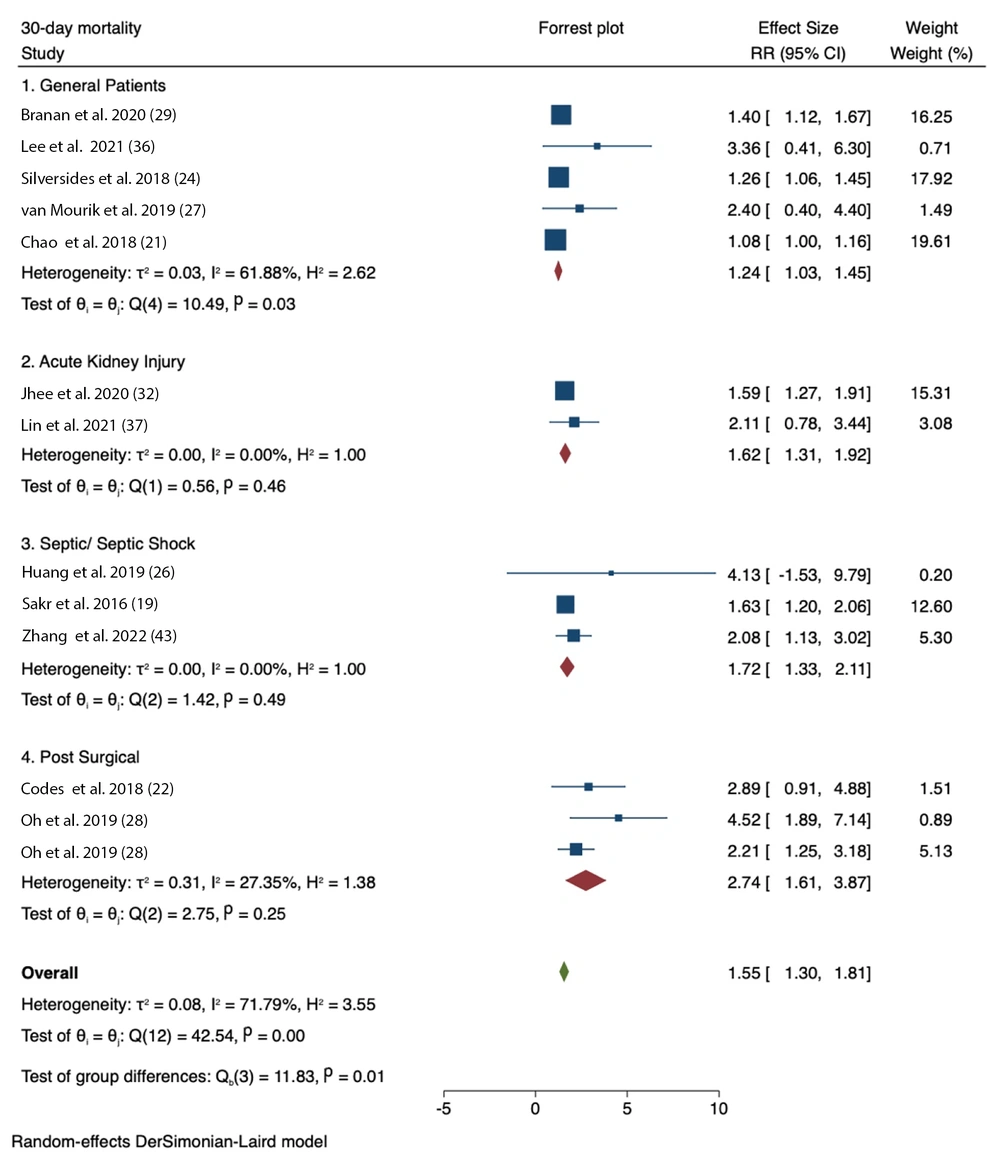
The analysis of positive CFB and 30-day mortality demonstrated significant associations across several patient groups: General patients (RR = 1.24, 95% CI = 1.03 - 1.45; I2 = 61.88%, P-heterogeneity = 0.03), patients with AKI (RR = 1.62, 95% CI = 1.31 - 1.92; I2 = 0.00%, P-heterogeneity = 0.46), patients with sepsis/septic shock (RR = 1.72, 95% CI = 1.33 - 2.11; I2 = 0.00%, P-heterogeneity = 0.49), and surgical patients (RR = 2.74, 95% CI = 1.61 - 3.87; I2 = 27.35%, P-heterogeneity = 0.25) (Figure 2).
Forest plots showing pooled RR with 95% CI of positive cumulative fluid balance (CFB) at any time point on 30-day mortality based on patient type. The squares indicate the estimated effect size and the weight of individual studies. Diamonds indicate the total effect sizes from all studies (19, 21, 22, 24, 26-29, 32, 36, 37, 43).
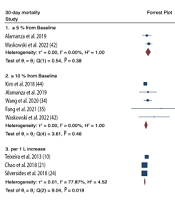
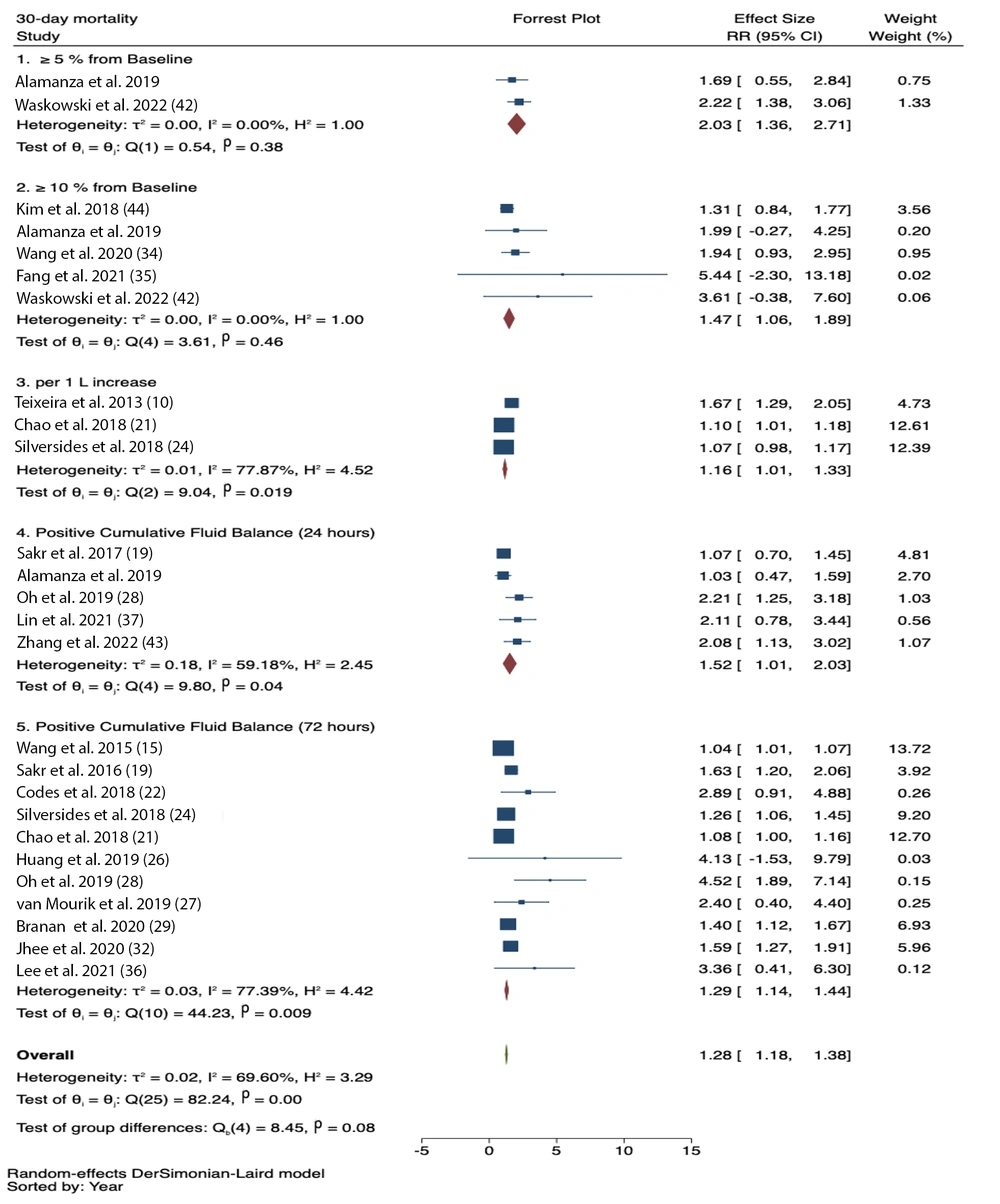
In the subgroup analysis based on FO assessment, significant associations were observed for FO ≥ 5% from baseline (RR = 2.03, 95% CI = 1.36 – 2.71; I2 = 0.0%, p-heterogeneity = 0.38), FO ≥ 10% from baseline (RR = 1.47, 95% CI = 1.06 – 1.89; I2 = 0.0%, p-heterogeneity = 0.46), first 24-hour positive CFB (RR = 1.52, 95% CI = 1.01 - 2.03; I2 = 59.18%, p-heterogeneity = 0.04), 72-hour positive CFB (RR = 1.29, 95% CI = 1.14 - 1.44; I2 = 77.39%, p-heterogeneity = 0.009), and per 1 L increase in CFB (RR = 1.16, 95% CI = 1.01 - 1.33; I2 = 77.87%, p-heterogeneity = 0.019) (Figure 3).
Meta-regression conducted on the 72-hour positive CFB subgroup showed that body weight, diabetes mellitus, hypertension, and cancer did not influence the results. The increase in mortality was significantly associated with older age (P-value < 0.001).
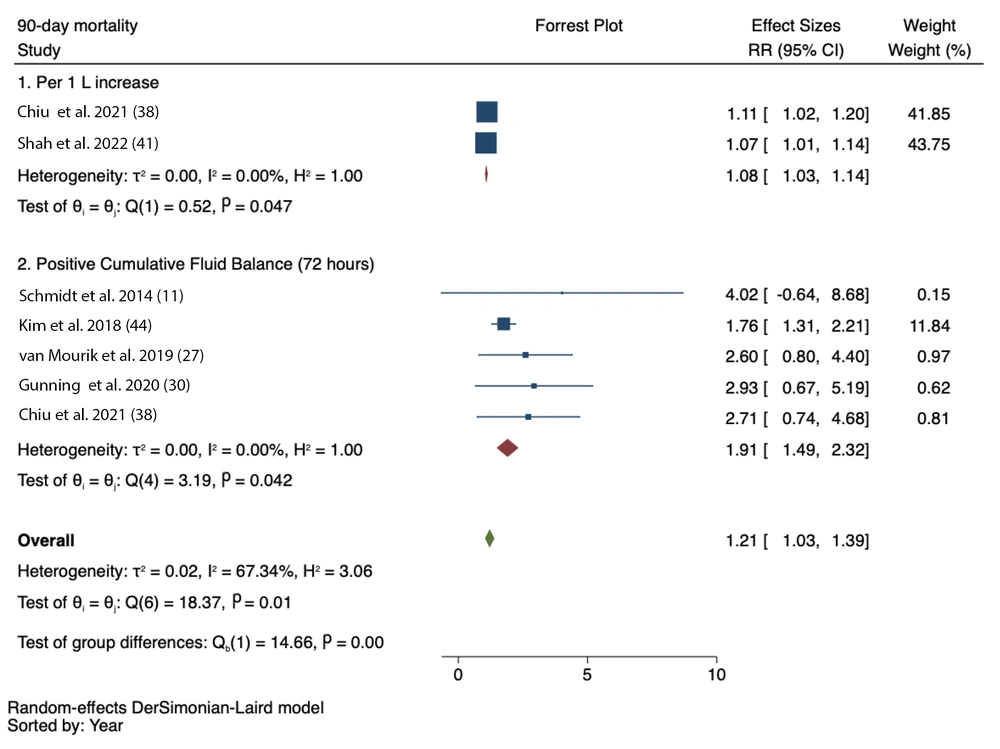
3.3. 90-day Mortality
Five studies reported a significant association between 72-hour positive CFB and 90-day mortality (RR = 1.91, 95% CI = 1.49 - 2.32; I2 = 0.00%, P-heterogeneity = 0.042). An increase in CFB per 1 litre was also associated with increased mortality within 90 days (RR = 1.08, 95% CI = 1.03 - 1.14; I2 = 0.00%, P-heterogeneity = 0.047) (Figure 4).
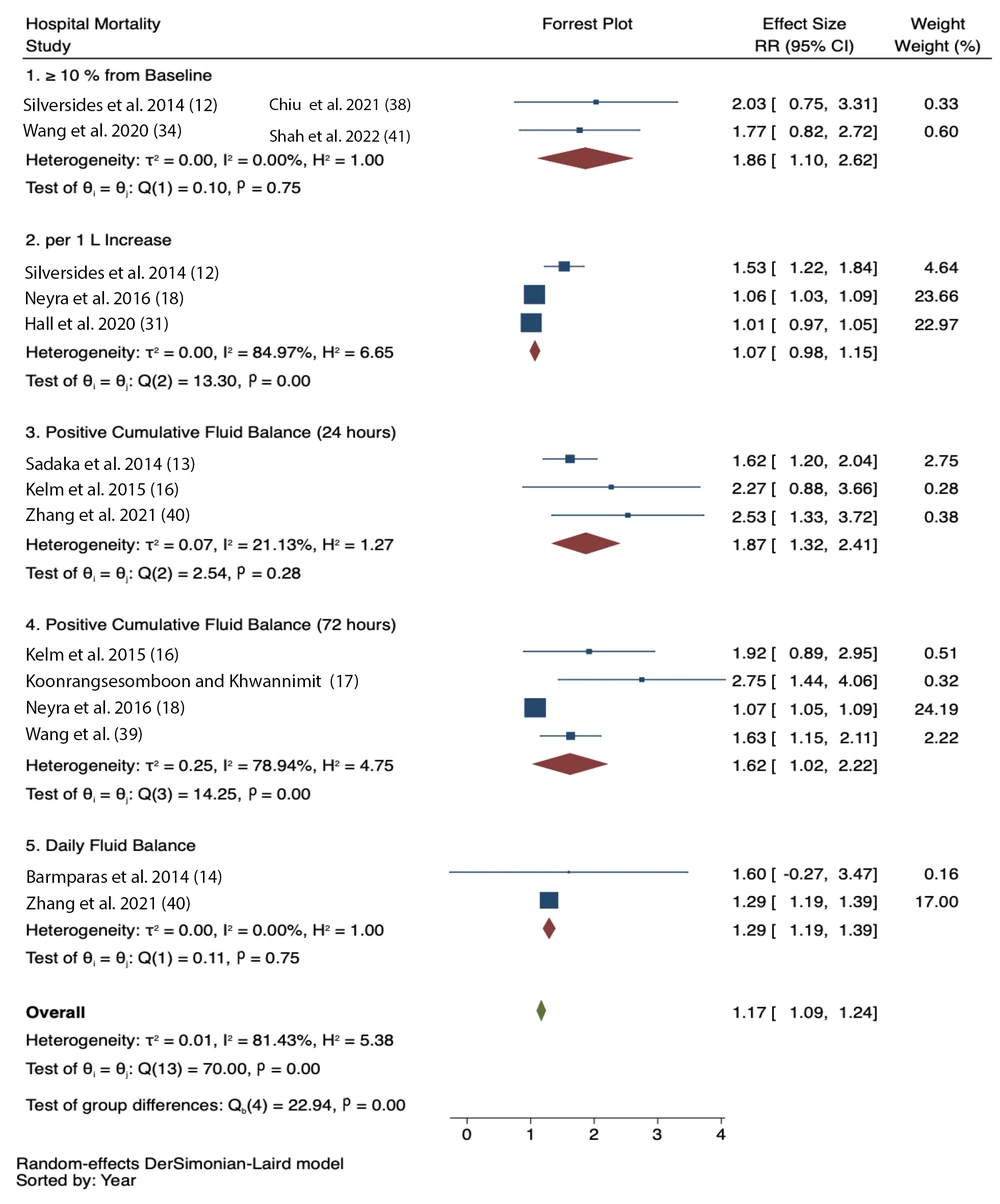
3.4. Hospital Mortality
Positive fluid balance was significantly associated with increased hospital mortality based on multiple assessments:
- 24-hour CFB (RR = 1.87, 95% CI = 1.32 - 2.41; I2 = 21.13%)
- 72-hour CFB (RR = 1.62, 95% CI = 1.02 - 2.22; I2 = 78.94%)
- FO ≥ 10% from baseline (RR = 1.86, 95% CI = 1.10 - 2.62; I2 = 0%)
- Daily fluid balance (RR = 1.29, 95% CI = 1.19 - 1.39; I2 = 0%)
However, CFB per 1 L increase did not show a statistically significant association with hospital mortality (RR = 1.07, 95% CI = 0.98 - 1.15; I2 = 84.97%) (Figure 5).
3.5. Publication Bias and Sensitivity Analysis
Sensitivity analysis using the leave-one-out method did not alter the statistical significance of the results (data not shown). For 30-day mortality, publication bias was suggested by Begg’s test (P = 0.08), Egger’s test (P = 0.04), and an asymmetrical funnel plot. A trim-and-fill analysis yielded a pooled RR of 1.056 (95% CI: 1.031 - 1.081) (Appendix 4 in Supplementary File). Similar asymmetry was noted for 90-day and hospital mortality (Appendices 5 and 6 in Supplementary File), with trim-and-fill analyses resulting in pooled RRs of 1.020 (95% CI: 1.010 - 1.029) and 1.064 (95% CI: 1.049 -1.079), respectively.
4. Discussion
Pooled adjusted risk estimates indicate that FO — whether based on body weight or CFB — is associated with increased mortality. Subgroup analysis revealed a significant association with 30-day mortality, particularly among patients with AKI and those who underwent surgery. In AKI patients, impaired fluid and electrolyte regulation, toxin accumulation, and increased oxidative stress may contribute to mortality through distant organ dysfunction (45).
Surgical patients experience hormonal and inflammatory responses that disrupt fluid balance, leading to water and sodium retention through the actions of antidiuretic hormone (ADH), aldosterone, and the renin-angiotensin system (RAS), with cortisol helping to maintain capillary integrity (46). Inflammatory mediators such as IL-6 and TNF, released in response to surgical trauma, further exacerbate fluid retention (47). Perioperative factors — including preoperative fluid deficits, anesthetic effects, and efforts to maintain urine output — often contribute to FO (48). This excess can impair cardiac function and result in pulmonary complications like edema and respiratory failure, ultimately increasing postoperative mortality.
Sepsis, particularly septic shock, involves a state of reduced blood flow caused by the body’s dysregulated response to infection and is associated with high rates of morbidity and mortality (16). Upon exposure to an infectious agent, both pro-inflammatory and anti-inflammatory immune responses are activated, involving complex interactions between white blood cells, inflammatory cytokines, and the endothelium (49). The endothelium, as the primary site of immune activation, undergoes microvascular injury and triggers both coagulation and complement cascades, which further exacerbate vascular damage and result in capillary leakage (50). This increases interstitial fluid accumulation, especially in the context of aggressive fluid administration.
This meta-analysis examined various methods of fluid excess assessment. Most studies measured CFB over the first 24 - 72 hours in the ICU, while only a few utilized daily fluid balance (14, 40). Although body weight measurement is considered a reliable indicator of fluid status, it is often impractical in critically ill patients, prompting clinicians to rely on fluid intake and output monitoring (8). However, daily fluid balance is susceptible to documentation errors, does not account for insensible losses, and is time-consuming (51). Furthermore, studies have shown that fluid balance does not always correlate with body weight changes, particularly in patients hospitalized for five days or more. Despite these limitations, CFB remains a commonly used method for estimating total body water in ICU settings (52).
Subgroup analysis showed that nearly all fluid excess assessment methods were generally associated with mortality. Nonetheless, some studies in this meta-analysis reported no significant associations (19, 25). One retrospective study on septic shock patients found that, after adjusting for illness severity and achievement of treatment goals, FO was not a significant predictor of mortality. This suggests that while FO may be more common in severely ill patients, its impact can be mitigated by early goal-directed therapy (25). Another study found no significant difference in mortality between 24-hour and 72-hour fluid balances, suggesting that fluid accumulation beyond the initial resuscitation phase may have a stronger link to adverse outcomes (19).
The association between fluid balance and mortality is largely due to increased atrial and venous pressures resulting from excessive fluid intake, which leads to tissue edema and impaired organ function. These pathophysiological mechanisms can ultimately result in multiple organ failure (53). The effect is more pronounced in encapsulated organs, which cannot accommodate excess fluid without increasing interstitial pressure — ultimately reducing perfusion and organ function (54). In the FEAST trial, excess fluid was linked to myocardial injury, which exacerbated circulatory collapse and led to death (55).
To date, debate continues regarding optimal fluid administration strategies for critically ill patients — specifically between restrictive strategies (which emphasize lower intravenous fluid volumes with early use of vasopressors) and liberal strategies (which prioritize fluid resuscitation before vasopressor initiation). So far, neither approach has shown a definitive mortality benefit (56).
This meta-analysis has several limitations. First, publication bias was indicated by asymmetrical funnel plots, although trim-and-fill analysis confirmed that results remained statistically significant. Second, variations in the definitions of FO across studies required subgroup analyses. Third, this study focused exclusively on mortality, limiting insights into other clinically relevant outcomes. Lastly, as with all observational studies, residual confounding may exist, which could lead to an overestimation of the observed effects. Further research is warranted to address these limitations.
5. Conclusions
Pooled adjusted risk estimates from this meta-analysis demonstrate that FO or positive CFB is associated with increased mortality. Patient survival outcomes depend on the balance between disease severity and achievement of treatment goals, regardless of the method used to assess fluid status or the strategy employed to manage it.