1. Background
Enlarged turbinates lead to persistent nasal obstruction, commonly seen in cases of vasomotor, infectious, and allergic rhinitis. To alleviate nasal obstruction caused by turbinate hypertrophy, surgical reduction is often necessary (1). Nasal surgical interventions may be prematurely terminated due to increased bleeding, making it critical for anesthesiologists to enhance intraoperative visibility and reduce bleeding (2).
Several medications are used effectively to achieve controlled hypotension during general anesthesia (GA), including α2-adrenergic agonists, direct vasodilators, beta-adrenergic blockers, inhalational anesthetics, prostaglandin E1, calcium channel antagonists, and adenosine (3). Dexmedetomidine (DEX) is a highly selective α-adrenoceptor agonist with greater affinity for the α2-adrenoceptor than clonidine, which explains its sedative and anxiolytic properties (4). Dexmedetomidine has a short redistribution half-life of six minutes and an elimination half-life of two hours, making it suitable for intravenous (IV) titration (5, 6). Its favorable effects include reducing the need for supplemental anesthetics and analgesics (7).
Dexmedetomidine induces vasoconstriction of peripheral blood vessels without significantly impacting overall cardiovascular function. This localized effect, achieved by activating α2-adrenoceptors on blood vessel walls, is advantageous in nasal surgeries for reducing bleeding (8).
Intranasal DEX is accessible, noninvasive, and practical, offering the same sedative and analgesic properties as the IV route (9, 10). Multiple clinical trials have shown that intranasal DEX, at doses of 1 - 1.5 µg/kg, significantly reduces intraoperative pain and induces drowsiness (6, 11, 12).
2. Objectives
This study aims to compare the efficacy of DEX-soaked nasal packing (NP) and IV DEX in controlling bleeding during turbinate surgeries.
3. Methods
This randomized, controlled, double-blind trial was conducted on 60 patients between June 2023 and March 2024.
3.1. Inclusion Criteria
Patients aged 18 - 65 years of both sexes, classified as ASA I or II, and scheduled for turbinate surgeries were included. Ethical approval was obtained from the Institutional Ethical Committee of Kafr-El Sheikh University Hospitals, Egypt (approval Code: KFSIRB200-3). All patients provided written informed consent. The trial was registered on clinicaltrials.gov (ID: NCT05911776).
3.2. Exclusion Criteria
Patients were excluded if they had a known allergy to DEX, second- or third-degree atrioventricular blocks, persistent bradycardia, coagulopathy, severe hepatic or renal impairment, blood disorders, were on anticoagulants, or were receiving chronic analgesic therapy.
3.3. Randomization and Blinding
Patients were randomly assigned to two groups (30 patients each) using computer-generated numbers and sealed opaque envelopes. Neither participants nor researchers were aware of group assignments. Drug preparation was performed by a pharmacist who was not involved in the study.
Group NP received DEX-soaked NP thirty minutes before surgery for ten minutes (1.5 𝜇g/kg intranasal DEX-soaked NP) and an IV bolus of saline over 10 minutes, followed by saline infusion.
Group IV received a 0.5 𝜇g/kg bolus of DEX over 10 minutes, followed by a 0.1 - 0.4 𝜇g/kg per hour IV infusion of DEX, along with saline-soaked NP (thirty minutes before surgery for ten minutes).
All patients underwent history-taking, clinical examination, and preoperative testing. They were trained to use the Visual Analog Scale (VAS) for pain, where 0 indicated no pain and 10 the highest level of pain.
Two venous cannulas were placed for administering DEX and saline infusions. An arterial cannula was inserted into the radial artery to continuously monitor blood pressure and draw blood samples. Routine monitoring included pulse oximetry, electrocardiogram, capnography, non-invasive blood pressure, and temperature.
General anesthesia was induced with fentanyl (1 - 2 µg/kg) and propofol (2 mg/kg). Cis-atracurium (0.15 mg/kg) was administered for muscle relaxation. Anesthesia was maintained with 1 - 1.5% isoflurane in O₂, with incremental doses of cis-atracurium (0.03 mg/kg). End-tidal CO₂ was kept between 30 - 40 mmHg by adjusting mechanical ventilation.
Intraoperative fentanyl doses (1 µg/kg) were administered when there was a 20% increase in MAP or HR. The total fentanyl dose, including that used for induction, was recorded. All patients were placed in a reverse Trendelenburg position (30° angle).
3.4. Data Recorded
HR and MAP were recorded at baseline and then every 10 minutes until the end of surgery, with all surgeries conducted by the same surgeon.
3.4.1. Intraoperative Blood Loss
Assessed by weighing gauze sponges and measuring the volume of blood suctioned into a column containing anticoagulants (13).
3.4.2. Surgical Field Quality
Evaluated using Formmer's 6-Point Scale (0 “no bleeding”; 1 “slight bleeding that did not hinder the surgery”; 2 “moderate bleeding that was an annoyance but not obstructive”; 3 “moderate bleeding that somewhat impacted the surgery”; 4 “severe but manageable bleeding that greatly interfered”; and 5 “extensive, uncontrollable bleeding”) (14).
3.4.3. Postoperative Pain Management
IV paracetamol (1 gr every 8 hours) was administered. Rescue analgesia was provided with IV pethidine (0.5 mg/kg) if the VAS exceeded 3. The time to the first request for analgesia and the total pethidine dosage in the first 24 hours were recorded.
3.4.4. Side Effects
Including postoperative nausea and vomiting (PONV), which was treated by combination therapy of 4 mg ondansetron IV followed by 4 mg every 8 hours and 8 mg of dexamethasone IV as a single dose; hypotension (defined as a MAP 20% below baseline and managed with ephedrine 5 mg IV bolus); and bradycardia (defined as HR below 60 beats per minute and treated with atropine 0.6 mg IV).
3.4.5. Patient Satisfaction
Assessed 24 hours post-surgery using a Five-Point Likert Scale ("very dissatisfied," "dissatisfied," "unsure," "satisfied," and "very satisfied").
The primary outcome was the amount of intraoperative blood loss, and the secondary outcomes were pain score, surgical field quality, and patient satisfaction.
3.5. Sample Size Calculation
The sample size was calculated using the PASS program (version 11.0; NCSS PASS, UT, USA). Intraoperative blood loss was the primary outcome of this non-inferiority study. The sample was determined by a group ratio of 1:1, a confidence interval of 95%, and a study power of 80%. The mean and standard deviation of intraoperative blood loss were found to be 29.43 mL in a prior investigation (15), and the non-inferiority margin was set to 20 mL. To combat technique failure and dropout, three cases were added to each group. Consequently, thirty individuals were enrolled in each group.
3.6. Statistical Analysis
Statistical analysis was conducted using SPSS v27 (IBM, Armonk, NY, USA). The Shapiro-Wilk test and histograms were employed to evaluate the normality of the data distribution. Mean and standard deviation (SD) were used to express quantitative parametric data, which were analyzed using the unpaired student t-test. The median and interquartile range (IQR) were used to express quantitative non-parametric data, which were analyzed using Mann-Whitney tests. The frequency and percentage were used to express qualitative variables, which were analyzed using the chi-square or Fisher's exact test. P-values less than 0.05 with two tails were deemed statistically significant.
4. Results
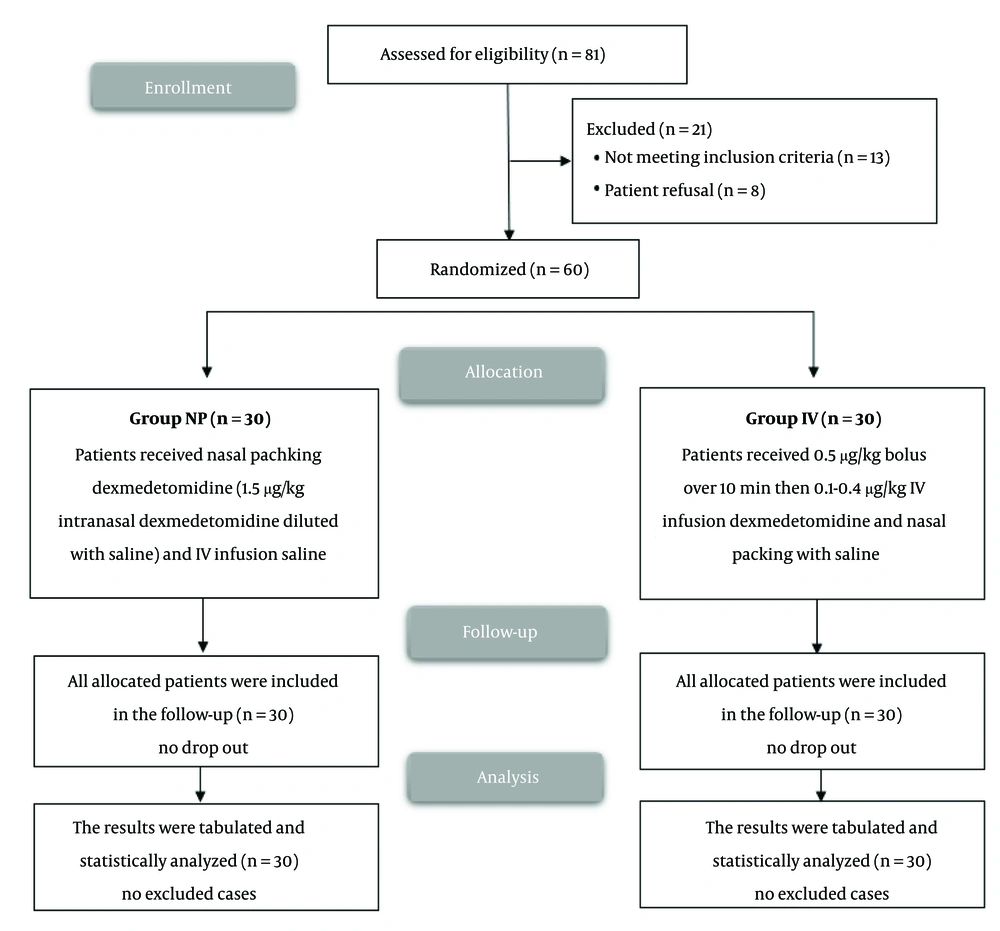
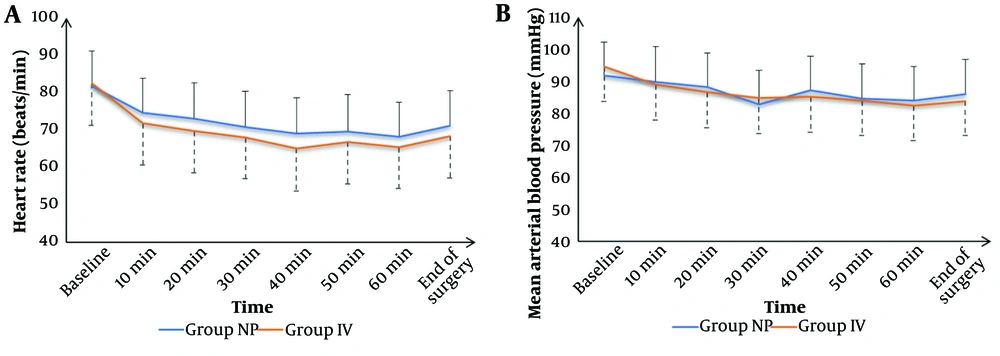
Of the 81 patients screened, 60 were randomized and completed the study (Figure 1). Patient demographics, including age, sex, and Body Mass Index (BMI), were similar between the two groups (Table 1). Heart rate (HR) and mean arterial pressure (MAP) measurements were also comparable across all time points, with no significant differences observed in the surgical field quality scores (Figure 2).
| Variables | Group NP (n = 30) | Group IV (n = 30) | P-Value |
|---|---|---|---|
| Age (y) | 38.7 ± 13.09 | 36.17 ± 10.13 | 0.405 |
| Sex | 0.426 | ||
| Male | 20 (66.67) | 17 (56.67) | |
| Female | 10 (33.33) | 13 (43.33) | |
| Weight (kg) | 72.27 ± 7.73 | 74.1 ± 6.64 | 0.328 |
| Height (cm) | 167.83 ± 7.81 | 168.17 ± 5.94 | 0.853 |
| BMI (kg/m2) | 25.67 ± 2.4 | 26.21 ± 2.1 | 0.354 |
| ASA physical status | 0.791 | ||
| I | 19 (63.33) | 18 (60) | |
| II | 11 (36.67) | 12 (40) | |
| Duration of surgery (min) | 78.33 ± 13.79 | 79.83 ± 7.93 | 0.608 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, Body Mass Index.
a Values are exprssed as mean ± SD or frequency (%).
The mean blood loss was 124.1 ± 34.85 mL in the NP group and 115.63 ± 31.89 mL in the IV group, showing no statistically significant difference (P = 0.330) (Table 2). Visual Analog Scale (VAS) scores and pethidine consumption were similarly comparable between the two groups at all time intervals (Tables 2 and 3). Patient satisfaction levels and side effects were also similar, with no significant differences between the NP and IV groups (Table 4).
| Variables | Group NP (n = 30) | Group IV (n = 30) | P-Value |
|---|---|---|---|
| Intraoperative blood loss (mL) | 124.1 ± 34.85 | 115.63 ± 31.89 | 0.330 |
| Formmer’s scores of surgical field quality | 3 (2 - 3) | 2.5 (2 - 3) | 0.375 |
| Time to first request rescue analgesia (h) | 8.93 ± 1.95 | 9.77 ± 1.72 | 0.084 |
| Total dose of pethidine consumption in the first 24 hours (mg) | 92.53 ± 29.06 | 85 ± 21.56 | 0.259 |
a Values are expressed as mean ± SD or median (IQR).
| Variables | Group NP (n = 30) | Group IV (n = 30) | P-Value |
|---|---|---|---|
| 2 h | 0 (0 - 1) | 0.5 (0 - 1) | 0.440 |
| 4 h | 2 (1 - 3) | 1 (1 - 2) | 0.215 |
| 6 h | 2.5 (1.25 - 3) | 2 (2 - 2.75) | 0.350 |
| 8 h | 3 (2 - 5) | 3 (2 - 4) | 0.500 |
| 12h | 2 (1.25 - 4.75) | 2 (1 - 4) | 0.779 |
| 18h | 5 (3 - 5) | 4 (3 - 4) | 0.172 |
| 24h | 4 (3 - 5) | 4 (3 - 4.75) | 0.673 |
a Values are expressed as median (IQR).
| Variables | Group NP (n = 30) | Group IV (n = 30) | P-Value |
|---|---|---|---|
| Patients’ satisfaction | 0.541 | ||
| Very satisfied | 12 (40) | 14 (46.67) | |
| Satisfied | 10 (33.33) | 12 (40) | |
| Unsure | 7 (23.33) | 4 (13.33) | |
| Dissatisfied | 1 (3.33) | 0 (0) | |
| Very dissatisfied | 0 (0) | 0 (0) | |
| Side effects | |||
| Bradycardia | 5 (16.67) | 7 (23.33) | 0.748 |
| Hypotension | 6 (20) | 10 (33.33) | 0.243 |
| PONV | 4 (13.33) | 3 (10) | 1 |
Abbreviation: PONV, postoperative nausea and vomiting.
a Values are presented as No. (%).
5. Discussion
Bleeding during nasal surgeries can hinder intraoperative visibility, so minimizing bleeding by maintaining controlled hypotension is essential (16). Dexmedetomidine can aid in controlling intraoperative bleeding due to its hemodynamic effects (17). Dexmedetomidine stimulates α-2 adrenergic receptors in blood vessels, causing vasoconstriction, which reduces the vessel diameter and decreases blood flow to the surgical site, thereby reducing bleeding (18).
In our study, the mean intraoperative blood loss was 124.1 ± 34.85 mL in the NP group and 115.63 ± 31.89 mL in the IV group. Although blood loss was lower in the NP group compared to the IV group, the difference was not statistically significant.
Kale et al. (19) reported that, during functional endoscopic sinus surgery (FESS), mean intraoperative blood loss after using 2 µg/kg of DEX was 135.06 ± 36.88 mL in the NP group. The DEX group experienced significantly less blood loss than the local anesthetic group. Similarly, Mohammed et al. (20) found that intranasal DEX (100 µg) significantly decreased intraoperative bleeding during FESS. In agreement, Wang et al. (21) demonstrated that blood loss during nasal endoscopic surgery was lower in the DEX groups (1 µg/kg and 2 µg/kg) than in the control group. Fazel et al. (22) also reported that intraoperative blood loss during FESS was lower in the DEX infusion group (0.2 µg/kg/h) than in the placebo group (252.7 ± 115 mL vs. 312.9 ± 83.9 mL). Additionally, Gousheh et al. (15) found that IV DEX (1.0 µg/kg given 10 minutes before GA induction, followed by 0.5 µg/kg/h infusion) reduced intraoperative blood loss compared to the control group (116.33 ± 29.43 mL vs. 250.69 ± 45.74 mL). Tang et al. (9) also noted that intranasal DEX significantly reduced intraoperative blood loss in patients undergoing FESS (29.2% vs. 33.8% in the placebo group). Furthermore, Ayoglu et al. (23) observed that IV DEX (1 µg/kg bolus, then 0.7 µg/kg/h maintenance) reduced bleeding in septoplasty procedures (52.7 ± 39.0 mL vs. 130.0 ± 73.1 mL in the control group).
In contrast, Huh et al. (24) reported a mean blood loss of 270 ± 116 mL in the DEX group. Patients in this study had an ASA physical status classification of III or IV, which may explain the difference.
Our results showed that hemodynamics, Formmer’s scores, time to first rescue analgesia, total pethidine consumption, VAS scores, patient satisfaction, and side effects were clinically comparable, with no statistically significant differences between the NP and IV groups.
Kale et al. (19) similarly found improved hemodynamics, surgical field quality, and patient satisfaction in the NP DEX group compared to the lignocaine-adrenaline group. Mohammed et al. (20) noted a significant reduction in pain scores and delayed time to first rescue analgesia in the intranasal DEX group. Consistent with our findings, Wang et al. (21) observed that DEX-soaked NP significantly relieved postoperative pain. Huh et al. (24) reported that DEX stabilized hemodynamic responses during endoscopic sinus surgery, with a surgical field score of 2 (2 - 2) and no significant adverse effects.
Fazel et al. (22) also found reduced pain intensity and pethidine consumption in the DEX infusion group compared to the placebo group. Gousheh et al. (15) reported lower total opioid consumption in IV DEX groups than in the control group. Tang et al. (9) similarly showed that hemodynamic variables, surgical field quality, pain scores, and patient satisfaction were improved in intranasal DEX groups compared to placebo. Ayoglu et al. (23) demonstrated reduced intraoperative opioid use with IV DEX in septoplasty operations.
5.1. Limitations
This study was limited by its small sample size, single-center design, and short follow-up period. Future studies with larger cohorts and control groups are needed to generalize these findings.
5.2. Conclusions
Dexmedetomidine NP is non-inferior to IV DEX for controlling bleeding during turbinate surgery. Both methods provided comparable surgical field quality, patient satisfaction, and pain management outcomes.