1. Background
Spirulina platensis is the dried biomass of the cyanobacterial genus Arthrospira spp., with the most common species being Arthrospira maxima, Arthrospira platensis, and Arthrospira fusiformis (1, 2). Spirulina is a type of blue-green algae (cyanobacterium) belonging to the kingdom Monera. Despite its classification as a cyanobacterium, it is often referred to as a plant by some botanists due to its ability to perform photosynthesis (3, 4). It is found in both fresh and marine waters and requires a pH close to 10 and a temperature between 35 - 37°C for optimal growth (5).
Spirulina consumption has an acceptable safety profile in both human and animal studies (6-8). Correspondingly, Spirulina has a toxicological safety profile and is listed by the US Food and Drug Administration under the category Generally Recognized as Safe (GRAS) (9). A typical safe dosing regimen for adults is considered to be approximately 3 - 10 g/day (10). Recent interest in Spirulina has grown significantly, even attracting the attention of the National Aeronautics and Space Administration (NASA) (11). Despite this emerging attention, the harvesting and consumption of Spirulina have been documented since the 16th century, particularly in Africa and the Aztec Empire (2, 12, 13).
Spirulina is a functional food with wide clinical applicability in nutrition due to its rich composition, which includes lipids, carbohydrates, hydrocarbons, sterols, proteins (containing 16 amino acids, including branched-chain amino acids), and several micronutrients (4, 5, 14). The nutritional composition of Spirulina per 100 g of dry weight is noteworthy: Three hundred seventy three kcal, 4.3 g total fat (1.95 g saturated fatty acids, 1.93 g polyunsaturated fatty acids, 0.26 g monounsaturated fatty acids), 17.8 g carbohydrates (7.7 g fiber, 1.3 g sugars), 63 g protein, 162 mcg vitamin B12, 468 mg calcium, 87.4 mg iron, 270 - 535 µg folate, 319 mg magnesium, 3.26 mg manganese, 1660 mg potassium, 25.5 mcg selenium, and 1.45 mg zinc (4, 14).
Spirulina supplements are widely available in powder or tablet form, with tablet doses generally ranging from 200 mg to 1000 mg—500 mg being the most common dose per tablet (15-19). Therapeutic dosing regimens of Spirulina vary from 0.8 - 10 g/day for durations ranging from 2 weeks to 6 months, according to meta-analyses of randomized clinical trials (20-28). Therefore, the macronutrient and micronutrient content across common Spirulina intake is not as significant as is often publicized.
Anti-inflammatory, antioxidant, and immunomodulatory properties are the primary well-studied attributes of Spirulina, while its antiviral effects are still emerging (29-31). The antiviral potential of Spirulina has already been investigated against influenza (H1N1), a viral respiratory disease that caused a pandemic in 2009 (32-34). A likely mechanism for Spirulina's action against virus-induced inflammatory responses involves the modulation of immune cells, which results in decreased levels of pro-inflammatory cytokines and enhanced antioxidant status (35).
In silico research suggests that Spirulina could be used against lower respiratory infections. More interestingly, Spirulina peptides are proposed to target the spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (36-38). In vitro studies support Spirulina's high antiviral activity against SARS-CoV-2 by inhibiting the secretion of tumor necrosis factor-alpha (TNF-α) (39, 40). Phycocyanobilins have been identified as a key component of Spirulina responsible for its immunomodulatory effects against SARS-CoV-2 (35, 41). Recently, it was found that Spirulina supplementation could improve certain laboratory markers (e.g., coagulation factors and lymphocytopenia) in intensive care unit (ICU) patients with coronavirus disease 2019 (COVID-19), although severity outcomes require further investigation as they are the cornerstone of the medical approach (42).
2. Objectives
Therefore, this study aimed to investigate the effects of Spirulina supplementation on disease severity scores, length of hospital and ICU stays, respiratory support at discharge, and 28-day mortality in ICU patients with COVID-19.
3. Methods
3.1. Study Design
This study was a single-center, double-blind, randomized controlled clinical trial with a parallel design. Participants were randomly assigned to two equal groups to evaluate the add-on effect of Spirulina platensis on 28-day mortality, hospital and ICU length of stay, and disease severity in adults with COVID-19. The study was conducted at Farhikhtegan Hospital, an educational general hospital in Tehran, Iran, from March 2021 to December 2021. The diagnosis of COVID-19 was confirmed using reverse transcriptase-polymerase chain reaction (RT-PCR) and findings consistent with COVID-19 pneumonia on computed tomography (CT). Patients or their legal representatives provided written informed consent for participation.
3.2. Study Population
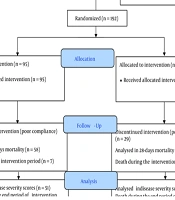
A total of 126 patients were enrolled in the study (Figure 1). The study included hospitalized patients with confirmed severe COVID-19 infection who were over 18 years old and had an oxygen saturation below 90% on room air at the time of admission. Exclusion criteria were: known Spirulina allergy, pregnancy, lactation, end-stage renal disease (ESRD), end-stage liver disease (cirrhosis), patients without a functioning gastrointestinal tract (contraindicating oral or enteral nutrition), and patients with an estimated survival of less than 24 hours. Data on underlying diseases, BMI, and concomitant medications were extracted from the patients' medical records. Laboratory evaluation data were collected twice: At baseline and at the end of the study.
The study protocol was approved by the ethical committee of the Islamic Azad University Faculty of Medicine (IR.IAU.PS.REC.1400.035 and IRCTID: IRCT20200720048139N1).
3.3. Randomization
Participants were randomly assigned in a 1:1 ratio to receive either Spirulina platensis supplementation (intervention group) or a placebo (control group) using a secure, centralized web-based system. An independent statistician provided a computer-generated randomization sequence with varying block sizes, which was unknown to the investigators.
3.4. Intervention
All patients in the intervention and placebo groups received the same standard treatment, which included oxygen therapy, antiviral agents (Remdesivir), antibacterial agents, corticosteroids, and anticoagulants. Spirulina platensis was provided by Drotat High-Tech and Pharmaceutical Science Company (Setare Shakhab Qeshm Co. Ltd, Iran) and administered orally at a dose of 5 g/day (an intermediate dose based on previous studies on other diseases) (10, 21). The intervention group received two capsules in the morning, two in the afternoon, and one at night with 240 mL of water (one glass). The capsules were distributed in two packets labeled A and B, so both the patients and the physician administering the capsules were blinded to the type of capsule being given.
Cornstarch was used as a placebo with the same dosing regimen. In some cases, for conscious patients, the capsules were opened, and the powder was dissolved in water before administration. For intubated patients, a gavage solution was used. Both the patients and the physician administering the capsules were blinded to the type of capsule.
The study treatment began within 24 hours after randomization and continued for 14 days. Site clinicians at the enrolling center screened ICU patients for eligibility. Eligible patients who consented to participate were randomized and followed for 14 days from randomization. While the patients were hospitalized, follow-up was conducted by the site clinicians who enrolled them. Upon hospital discharge, follow-up was performed through daily telephone calls by the same site clinicians.
3.5. Study Outcome
The four primary outcomes of the study were: (1) ICU length of stay, (2) hospital length of stay, (3) proportion of patients requiring oxygen support after discharge, and (4) 28-day all-cause mortality.
Secondary outcomes included severity indexes: Acute Physiology and Chronic Health Evaluation II (APACHE II), National Early Warning Score (NEWS) 2, Nutrition Risk in Critically Ill (NUTRIC), and Sequential Organ Failure Assessment Score (SOFA). The SOFA score was developed as a straightforward and effective tool to characterize organ failure in critically ill patients, with data initially based on 40 ICUs across 16 countries (43). As a practical method, the SOFA score allows for the calculation of individual or aggregate organ dysfunction severity across multiple systems (respiratory, coagulation, liver, cardiovascular, renal, and neurological), with scores ranging from 0 to 24 points. A higher SOFA score indicates worse organ function (44). These forms were completed at the beginning and end of the study.
3.6. Statistical Methods
The sample size was calculated considering an α of 0.05 (type I error) and a β of 20% (type II error). Since no similar studies on this disease were available, the effect size (d) was assumed to be 0.5 (medium). Therefore, the minimum required sample size was estimated to be 51 participants per group. The Kolmogorov-Smirnov test was used to determine if the data followed a normal distribution. Continuous and categorical variables were expressed as the median (with interquartile range) and percentages, respectively. Repeated measures ANOVA and linear regression models were used to analyze between-group differences. Additionally, logistic regression was applied to compare binary variables between groups. The repeated measures ANOVA examined the effects of time, intervention, and the interaction between intervention and time. All linear and logistic models were adjusted for age, sex, Body Mass Index (BMI), and baseline levels of the outcome variable. Kaplan-Meier curves and the log-rank test were used for two-group comparisons to analyze cumulative survival. Cox proportional hazards regression, with the proportional hazard assumption, was employed to adjust for covariate effects associated with 28-day mortality. Statistical analyses were performed using IBM SPSS 23.0, and a P-value of ≤ 0.05 was considered statistically significant. EndNote X8 was used to manage references.
4. Results
First, 230 patients were assessed for eligibility between March 1, 2021, and December 6, 2021. Of these, 38 patients did not meet the inclusion criteria. The remaining 192 patients were randomly divided into two groups: Ninety-seven participants in the Spirulina group and 95 in the placebo group. A total of 126 participants were included in the survival analysis. Sixty-six participants (29 in the Spirulina group and 37 in the control group) discontinued the intervention due to poor compliance. Their characteristics are shown in Table 1. Twenty-two participants were excluded from the secondary outcome analysis during the intervention due to death. After accounting for deaths from the end of the intervention to hospital discharge, 87 participants were analyzed for hospitalization duration and respiratory support status at discharge, with 46 participants in the Spirulina group and 41 in the control group (Figure 1).
| Characteristics | Control (n = 58) | Spirulina (n = 68) | P-Value |
|---|---|---|---|
| Age (y) | 56 (49.75 - 67.25) | 59 (49.5 - 66.5) | 0.64 |
| Ranges | 22 - 86 | 27 - 86 | |
| Male (participants) | 30 (51.7) | 43 (63.2) | 0.19 |
| BMI (kg/m2) | 27.32 (25.50 - 31.28) | 27.76 (25.4 - 31.76) | 0.78 |
| BMI categories | 0.91 | ||
| < 25 | 12 (20.7) | 14 (20.6) | |
| 25 - 30 | 30 (51.7) | 33 (48.5) | |
| +30 | 16 (27.6) | 21 (30.9) | |
| NEWS (score) | 8 (7 - 9) | 8 (7 - 10) | 0.75 |
| APACHE (score) | 17 (15.75 - 19) | 18 (15 - 20) | 0.42 |
| SOFA (score) | 6 (5 - 7.25) | 6 (5 - 7) | 0.20 |
| NUTRIC (score) | 4 (3 - 5) | 4 (3 - 5.75) | 0.53 |
| Comorbidities | |||
| Diabetes | 10 (17.2) | 15 (22.1) | 0.49 |
| Hypertension | 21 (36.2) | 30 (44.1) | 0.36 |
| Heart disease | 12 (20.7) | 17 (25) | 0.56 |
| Pulmonary disease | 0(0) | 1 (1.5) | 0.35 |
| Dyslipidemia | 5 (8.6) | 8 (11.8) | 0.56 |
| Laboratory markers | |||
| CRP (mg/L) | 95.55 (47.87 - 150) | 92.35 (38.10 - 141.50) | 0.29 |
| Neutrophil | 83 (75 - 88) | 85 (79.25 - 88) | 0.43 |
| Lymphocyte | 12 (7 - 20) | 10 (7 - 17) | 0.40 |
| Serum creatinine (mg/dL) | 1.1 (0.9 - 1.3) | 1.0 (0.8 - 1.1) | 0.06 |
| Bilirubin level (mg/dL) | 0.9 (0.6 - 1.1) | 0.8 (0.6 - 1.0) | 0.48 |
| Platelet count (µL) | 197,500 (165,750 - 264,500) | 194,500 (148,000 - 234,500) | 0.38 |
| Ratio of PaO2 to FIO2 | 61 (47 - 87) | 65 (48 - 78) | 0.61 |
| Glasgow Coma Scale score | 15 (14 - 15) | 15 (14 - 15) | 0.90 |
| Mean arterial pressure (mm Hg) | 9.24 (8.60 - 9.90) | 9.57 (8.91 - 34.93) | 0.21 |
| D-dimer (ng/mL) | 795 (554 - 1848) | 963 (596 - 1874) | 0.36 |
| Concomitant medications | |||
| Antibacterial agents | 53 (91.4) | 64 (95.5) | 0.34 |
| Anticoagulants | 48 (82.8) | 56 (82.4) | 0.95 |
| Antiviral agents | 30 (51.7) | 36 (52.9) | 0.89 |
| Corticosteroids | 29 (50.0) | 38 (55.9) | 0.51 |
Abbreviations: APACHE, acute physiology and chronic health evaluation; NEWS, National Early Warning Score; SOFA Score, Sequential Organ Failure Assessment Score; NUTRIC, nutrition risk in critically ill.
a P-value calculated using chi-square test for categorical data and Mann-Whitney U test for continuous data.
b Values are expressed as median (interquartile range) or No. (%).
There were no between-group differences in age, sex, BMI, comorbidities, severity indexes (NEWS2, APACHE, SOFA, and NUTRIC scores), use of medications, or baseline prognostic biomarkers (Table 1).
Table 2 provides disease severity scores at baseline and at the end of the intervention for both the control and intervention groups. All scores decreased significantly at the study's endpoint in both groups compared to baseline (within-group difference). However, only the SOFA score showed a significant decrease in the Spirulina group compared to the control group (P-value = 0.03). The NEWS2, APACHE, and NUTRIC scores did not show a significant change in the Spirulina group compared to the control group (P-values = 0.76, 0.88, and 0.77, respectively).
| Variables | Control | Spirulina (n = 53) | P-Value b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | End | n | Baseline | End | Time | Group | Time × Group | |||
| NEWS score | 50 | 8.20 ± 1.87 | 6.34 ± 1.58 | -1.86 ± 2.48 | 53 | 8.49 ± 2.29 | 5.96 ± 1.40 | -2.52 ± 2.74 | 0.12 | 0.76 | 0.16 |
| APACHE Score | 49 | 16.98 ± 3.15 | 14.55 ± 3.71 | -2.42 ± 4.35 | 53 | 17.04 ± 2.84 | 14.64 ± 3.56 | -2.39 ± 3.58 | 0.54 | 0.88 | 0.82 |
| SOFA score | 51 | 6.56 ± 1.48 | 5.08 ± 1.29 | -1.49 ± 1.94 | 53 | 6.32 ± 1.25 | 4.64 ± 1.03 | -1.67 ± 1.72 | 0.53 | 0.03 | 0.63 |
| NUTRIC score | 49 | 3.86 ± 1.32 | 2.98 ± 1.23 | -0.87 ± 1.33 | 53 | 4.02 ± 1.88 | 2.89 ± 1.15 | -1.13 ± 1.61 | 0.29 | 0.77 | 0.54 |
a Values are expressed as mean ± SD.
b Repeated ANOVA models (P < 0.05) used for between-group comparison and adjusted for age, gender, and Body Mass Index.
For all subjects combined, the ICU and hospital lengths of stay were 8.64 ± 4.49 days and 12.87 ± 4.27 days, respectively (Table 3). The Spirulina group had a shorter ICU stay compared to the control group (7.43 ± 3.98 days vs. 10.00 ± 4.69 days; P-value = 0.007) as well as a shorter hospital stay (11.48 ± 3.63 days vs. 14.44 ± 4.44 days; P-value = 0.001). At discharge, 34.1% of participants in the control group and 28.3% in the Spirulina group required oxygen support; however, there was no significant difference between the groups (P-value = 0.54) (Table 3).
| Variables | All (n = 87) | Control (n = 41) | Spirulina (n = 46) | P-Value Between Groups |
|---|---|---|---|---|
| Number of days in the ICU | 8.64 ± 4.49 | 10.00 ± 4.69 | 7.43 ± 3.98 | 0.007 |
| Number of days in the hospital | 12.87 ± 4.27 | 14.44 ± 4.44 | 11.48 ± 3.63 | 0.001 |
| O2 support (%) after discharge | 27 (31.0) | 14 (34.1) | 13 (28.3) | 0.54 |
a Values are expressed as Mean ± SD or No. (%).
b Linear regression for continuous variables and logistic regression for binary variables (P < 0.05); all regression models were adjusted for age, gender, body mass index, and NEWS score
The mortality rate was 22 patients in the Spirulina group and 17 patients in the control group. Kaplan-Meier analysis found no significant differences in survival distributions between the Spirulina and control groups (P = 0.97, log-rank test) (Figure 2). After adjusting for relevant confounding factors (age, BMI, and NEWS2 score), the multivariable Cox proportional hazards model showed no significant effect of Spirulina on 28-day survival [Hazard Ratio = 1.03, 95% Confidence Interval 0.55 - 1.89, P = 0.93].
5. Discussion
This study investigated the effects of Spirulina supplementation compared to a placebo in adults with COVID-19 who were admitted to the ICU. Spirulina supplementation reduced both hospital and ICU length of stay. However, the results showed no significant effect of Spirulina supplementation on 28-day mortality compared to the control group (between-group analysis), and most indexes of severity (NEWS2, APACHE, and NUTRIC scores) did not change, although the SOFA score was reduced.
Spirulina-based nutraceuticals are thought to enhance adaptive and innate immunity and exert antiviral effects due to a variety of bioactive compounds such as angiotensin-converting enzyme inhibitor peptides, sulfated polysaccharides, calcium-Spirulan, and phycobiliproteins (45). Notably, phycocyanobilins have been identified as the main compounds responsible for the immunomodulatory and antiviral activity of Spirulina, suggesting they could be the primary Spirulina component effective against severe COVID-19 (35, 41).
The daily dose of Spirulina (5 g) used in this study contains about 33 mg of phycocyanobilin, but a higher dosage might be necessary to achieve optimal therapeutic effects. For example, a triple dose (15 g, or one tablespoon) of the Spirulina regimen contains about 100 mg of phycocyanobilin, which could be a reasonable target for therapeutic intervention in humans, as 70 - 460 mg of phycocyanobilins are estimated to produce clinical effects in a 70 kg human (46). Phycocyanobilins mimic biliverdin in activating anti-inflammatory signaling pathways mediated by biliverdin reductase (47). More specifically, phycocyanobilins act as potent inhibitors of NADPH oxidase activity (48), an enzyme complex involved in severe COVID-19 that generates reactive oxygen species and contributes to oxidative stress (48).
Apart from the modulation of NADPH oxidase, potential mechanisms for the antiviral action of Spirulina appear to involve the production of B lymphocytes, activation of macrophages and natural killer cells, T cell proliferation, and cytokine modulation (35). While the COVID-19-induced cytokine storm is a recognized concern regarding COVID-19 severity (49, 50), Spirulina has inhibitory potential in the production of pro-inflammatory cytokines such as interleukin 6 (IL-6) and TNF-α, along with increasing antioxidant status (51-53).
The SOFA score has primarily been used to monitor patients with sepsis (54) and, more recently, has gained attention in severe COVID-19 cases to enhance individual care and responses to emerging therapies (55, 56). Despite all expectations regarding the SOFA score, it is important to note that the significant between-group difference in the SOFA score we observed may not necessarily reflect clinical relevance, even though statistical significance was achieved for both the control and Spirulina groups. Therefore, the shorter hospital and ICU length of stay for the Spirulina supplementation group, compared to the placebo group, is the main favorable result of our study, as it shows both statistical and clinical significance. Reducing hospital and ICU length of stay is a valuable outcome, improving patient welfare and reducing treatment costs, which tend to be high due to the necessary management of critical illness (57).
Given the lack of animal and human studies on the effects of Spirulina supplementation on SARS-CoV-2 before our study, an indirect focus on other viruses may provide a physiological rationale. In H1N1-infected rats, Spirulina supplementation had a dose-dependent effect on mortality, with doses of 0, 10, 25, or 50 mg/kg for 4 days after infection resulting in survival rates of 0%, 20%, 40%, and 60%, respectively, during a 2-week observation period (34). In another study involving H1N1-infected rats, Spirulina supplementation at 10 - 12 mg/d for 30 days before and 21 days after H1N1 infection reduced clinical symptoms of the ailment, pulmonary histopathology scores, and weight loss, while increasing appetite compared to the control group (33).
As for strengths, this study is novel in that it specifically examined patients suffering from severe COVID-19, as confirmed not only by clinical scores but also by laboratory markers, such as elevated D-dimer and CRP levels, which suggest a cytokine storm as a result of COVID-19. However, the study has some limitations. We did not investigate the entire signaling pathway of severe COVID-19, which involves several cytokines, including IL-1, IL-6, and TNF-α. Although these biomarkers are important for elucidating pathophysiological and molecular mechanisms (58-60) in medicine and nutrition, focusing on clinical scores can provide more specific and pragmatic conclusions.
Importantly, we did not find differences in comorbidities (such as diabetes, pulmonary disease, heart disease, and dyslipidemia), medication use (antiviral and antibacterial agents, corticosteroids, and anticoagulants) at baseline, or prognostic markers such as CRP, D-dimer, and the ratio of PaO2 to FIO2. Although controlling for these baseline values seems basic, some studies in nutrition and medicine have failed to do so (61, 62), which can lead to an overestimation of the effects of supplements or pharmacological agents.
Considering the importance of nutritional approaches in managing COVID-19 and critical care, Spirulina supplementation could be considered an adjuvant therapy for COVID-19. The effects of any nutritional strategy in COVID-19 are not indisputable and are not a substitute for drug therapies. However, further attention to Spirulina supplementation and its phytonutrients might be reasonable as a means of complementing personalized dosing regimens of vitamins, minerals, and macronutrients. Finally, further research is crucial to understanding the effects of other dosing regimens of Spirulina, particularly higher doses (e.g., 6 - 15 g/d) than we studied, in critically ill COVID-19 patients.
Spirulina supplementation effectively reduced the SOFA score and shortened hospital and ICU stays in critically ill patients with COVID-19. However, the supplementation did not improve NEWS2, APACHE, or NUTRIC scores, respiratory support at discharge, or 28-day mortality. Thus, Spirulina supplementation might be considered an adjunctive approach to standardized medical treatment in critically ill COVID-19 patients, but caution is necessary to avoid overvaluing its effects. Lastly, further research is needed to ascertain whether our findings will be replicated. Equally important, future studies focusing on the effect of Spirulina supplementation on mild to moderate COVID-19 are crucial to increasing the evidence.