1. Background
Since 1899, when August Bier performed the first spinal anesthetic procedure in Germany, spinal anesthesia has remained the gold standard for regional anesthesia in lower extremity surgeries. Over the years, various drugs have been utilized for spinal anesthesia, with bupivacaine being the most widely used local anesthetic since its introduction to clinical practice in 1965 (1).
Bupivacaine is commonly used alone to induce spinal anesthesia. However, it is standard practice to combine an opioid with a local anesthetic during intrathecal blocks to enhance anesthesia quality and provide effective postoperative pain relief. Local anesthetics work by stabilizing neuronal membranes, while opioids are believed to inhibit neuronal excitation within the spinal cord itself (1-3).
Pethidine (meperidine) is a unique synthetic opioid agonist with both analgesic and notable local anesthetic properties, allowing it to be used as a standalone medication for spinal anesthesia. Intrathecal pethidine may offer a low-cost alternative to conventional anesthetics for providing spinal anesthesia and analgesia in lower limb surgeries. This can be especially valuable for patients with allergies to ester or amide local anesthetics (4).
Lewis et al. evaluated the use of pethidine as the sole intrathecal anesthetic for transurethral resection of the prostate, reporting favorable outcomes compared to bupivacaine, which established beneficial conditions for pain control during lower abdominal and pelvic surgeries. Other studies have shown that 1 mg/kg of intrathecal pethidine provides effective surgical anesthesia, with prolonged postoperative analgesia and quicker motor recovery compared to intrathecal bupivacaine. However, the effects of pethidine as a sole intrathecal agent have not been extensively explored in recent studies (5-7).
Dexamethasone holds a prominent role in modern anesthesia practice, notably for reducing the incidence of postoperative nausea and vomiting. It is also used as an adjuvant to local anesthetics in spinal and other regional anesthesia to enhance the anesthetic profile and extend postoperative analgesia. Studies have shown that intrathecal dexamethasone can help reduce spinal anesthesia-related hypotension, shivering, and nausea (8-13).
2. Objectives
We hypothesized that combining intrathecal dexamethasone with pethidine might serve as an effective alternative to bupivacaine for spinal anesthesia in distal lower extremity surgeries, potentially resulting in improved outcomes and fewer adverse effects.
3. Methods
3.1. Study Design and Population
This prospective randomized double-blind study was designed following the guidelines and regulations of the Helsinki Declaration and received approval from our Institutional Review Board (ZU-IRB# 8035/7-11-2021). The study protocol was registered on clinicaltrials.gov (Ref: NCT05303311, registration date: January 15, 2022), with the enrollment of the first patient beginning on February 1, 2022.
The study was conducted in the orthopedic theaters of Zagazig University Hospitals from February 2022 to March 2024. It included 46 male and female participants aged over 18 years with a Body Mass Index (BMI) between 18.5 - 30 kg/m² and classified as ASA I or II by the American Society of Anesthesiologists physical status criteria. These participants were scheduled for elective lower extremity orthopedic surgery under spinal anesthesia. All patients provided written informed consent after being fully briefed on the study's purpose.
Patients were excluded from the study if they were uncooperative, had altered mental status, a known allergy to the study drugs, contraindications to spinal anesthesia, a history of epilepsy, chronic opioid use, were currently on antidepressants, or had severe respiratory, hepatic, or renal dysfunction.
During preoperative preparation, the study's goals and endpoints were thoroughly explained to the participants, and the numerical pain rating score (NRS) (0 indicating no pain and 10 indicating the worst pain) was introduced. A physical examination and review of laboratory investigations were completed. Fasting was confirmed with a requirement of 2 - 4 hours for clear fluids and 6 hours for solid food.
During the intraoperative period, standard monitoring was applied to all participants, including pulse oximetry, ECG, and noninvasive blood pressure, with baseline parameters recorded. An 18-gauge intravenous (IV) cannula was inserted, and each patient was preloaded with 500 mL of Ringer’s lactate. The 46 patients were then randomly assigned to two groups using simple randomization via a computer-generated table, with even numbers representing the control group and odd numbers representing the intervention group:
(1) Group PD (n = 23): Patients in this group received spinal anesthesia via a lumbar puncture performed with a 25-gauge BD® Quincke Needle in the sitting position at the L3-4 interspace. The intrathecal injection consisted of a mixture of 1 mg/kg preservative-free pethidine (Pethidine Injection 5%, equivalent to 50 mg/mL, "Misr Company for Pharmaceuticals," Egypt) and 4 mg dexamethasone (Dexamethasone Sodium Phosphate 0.4%, equivalent to 4 mg/mL, "Amirya Pharmaceutical Industries," Egypt), diluted with 0.9% sodium chloride to a total volume of 3 ml.
(2) Group B (n = 23): Patients in this group received spinal anesthesia via a lumbar puncture performed with a 25-gauge BD® Quincke Needle in the sitting position at the L3-4 interspace. The intrathecal injection consisted of 3 mL (15 mg) hyperbaric bupivacaine 0.5% alone (Bupivacaine 0.5%, "Sunnypivacaine," equivalent to 5 mg/mL, "Sunny Pharmaceutical Industries," Egypt).
During the intraoperative period, sensory and motor levels were assessed every minute for the first 10 minutes, then at 2-minute intervals until a stable block was achieved, at which point surgery was permitted to proceed. The speed of onset of the block was documented. These assessments continued at 15-minute intervals until sensory sensation returned to the 5th lumbar dermatome, and full motor function was restored.
Sensory block was tested by observing the loss of sensation to pinprick, while motor block was evaluated using the Modified Bromage Score: 0 indicated full leg movement, 1 indicated the inability to raise the leg against gravity but the ability to bend the knee and ankle joints, 2 indicated the inability to flex the hip and knee joints but not the ankle, 3 indicated the inability to flex the hip, knee, and ankle joints but the ability to move the toes, and 4 indicated full leg paralysis. Intraoperative sedation was provided with midazolam at 0.05 mg/kg as needed.
Postoperative pain control was managed with acetaminophen (1 g every 8 hours) and ibuprofen (400 mg IV every 6 hours). Parenteral morphine (2.5 - 10 mg) was used as rescue analgesia when the Numerical Rating Scale (NRS) was above 4. If mean arterial blood pressure dropped by 20% from baseline, 5 mg of IV ephedrine was administered. Symptomatic bradycardia (heart rate < 50 beats/min) was treated with 0.5 - 1 mg of IV atropine as required, and supplemental oxygen was provided if SpO2 fell below 92%.
The incidence of intraoperative and postoperative nausea, vomiting, and pruritus was documented. In cases of intolerable pruritus, 0.1 mg of naloxone was administered. Both the patient and the anesthesiologist collecting data were blinded to the study groups.
3.2. Study Outcome Measures
(1) Time to first need for rescue analgesia: Defined as the interval from the end of the intrathecal injection of the study drugs to the first patient-reported pain reaching an NRS of 4.
(2) Characteristics of spinal anesthesia:
- Onset of sensory block at the T10 dermatome.
- Onset of motor block, reaching a Bromage score of 4.
- Time for regression of sensory block to the 5th lumbar dermatome.
- Time for regression of motor block to full motor function (Bromage score 0).
(3) Intraoperative hemodynamics: Incidence of hypotension, defined as a mean arterial blood pressure decrease of > 20% from baseline, or bradycardia, defined as a reduction in heart rate > 20% of the baseline reading.
(4) Incidence of perioperative adverse events: Includes occurrences of nausea, vomiting, sedation, shivering, pruritus, and respiratory depression.
3.3. Sample Size
A pilot study was conducted on 10 patients in each group, revealing that the Mean ± SD time to the first need for rescue analgesia was 5.4 ± 0.8 hours in the Bupivacaine group and 6.5 ± 1.38 hours in the Pethidine plus Dexamethasone group, with an alpha error (α = 0.05) and a beta error (β = 0.10). Using OpenEpi, the calculated sample size was 23 patients per group.
3.4. Statistical Analysis
Data were collected, coded, and analyzed using the Statistical Package for the Social Sciences (SPSS software, version 20.0). Qualitative data were presented as numbers and percentages, while quantitative data were expressed as mean ± SD. Appropriate tests were used to assess the significance of differences: The chi-square test (X²) was applied for differences and associations in qualitative variables, while the t-test or Mann-Whitney test was used for differences between independent quantitative variables. Paired variables were analyzed using the paired t-test as appropriate. A P-value of < 0.05 was considered statistically significant.
4. Results
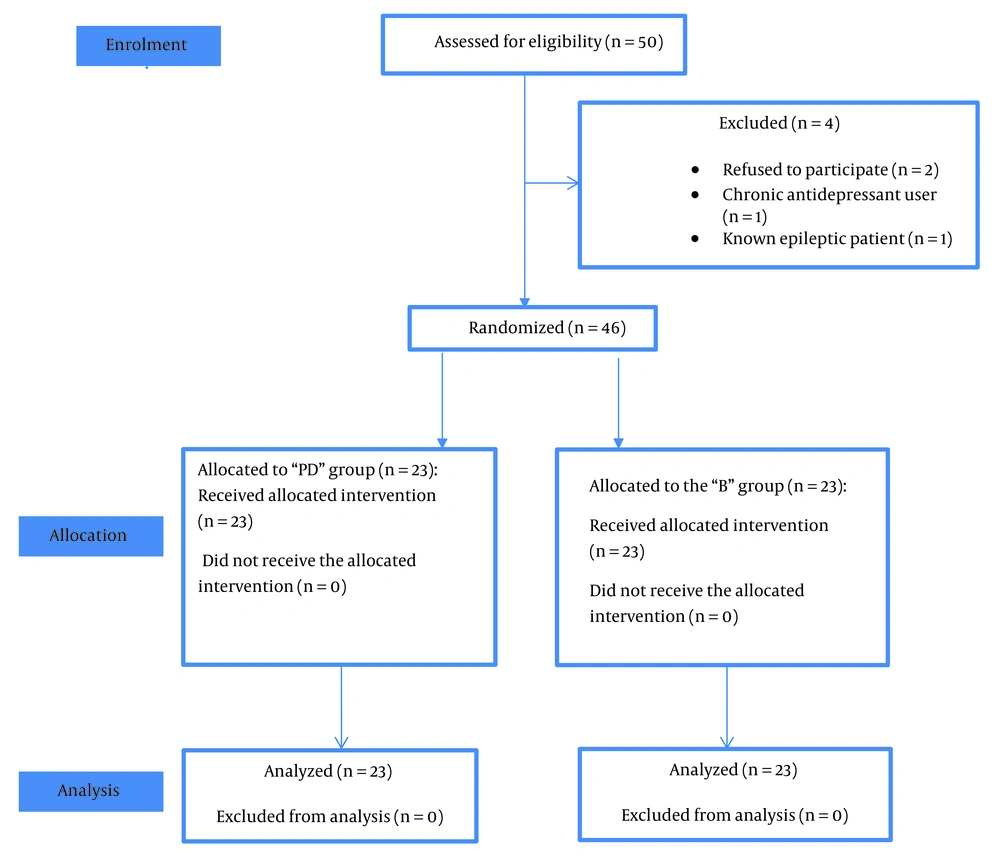
Fifty patients scheduled for elective distal lower limb orthopedic surgeries were enrolled in the study. Four patients were excluded (2 refused to participate, 1 was a chronic antidepressant user, and 1 was a known epileptic patient). The remaining forty-six patients were equally and randomly assigned to either the PD or B groups (Figure 1).
There was no significant difference between the study groups in terms of gender, age, BMI, ASA physical status, or the duration and type of surgeries (Table 1).
| Variables | PD Group; (N = 23) | B Group; (N = 23) | t | P-Values b |
|---|---|---|---|---|
| Gender | 0.088 | 0.767 | ||
| Female | 11 (47.8) | 10 (43.5) | ||
| Male | 12 (52.2) | 13 (56.5) | ||
| Age (y) | 44.26 ± 11.08 | 43.61 ± 11.56 | 0.195 | 0.846 |
| BMI (kg/m2) | 29.52 ± 2.84 | 29.26 ± 2.63 | 0.323 | 0.748 |
| ASA | 0.09 | 0.77 | ||
| I | 13 (56.5) | 14 (60.9) | ||
| II | 10 (43.5) | 9 (39.1) | ||
| Duration of surgery (min) | 68.83 ± 9.43 | 69.61 ± 9.89 | 0.275 | 0.785 |
| Types of Surgery | 2.933 | 0.820 | ||
| Achilles tendon repair | 3 (13.04) | 1 (4.35) | ||
| K-wire fixation of the ankle | 2 (8.70) | 4 (17.39) | ||
| Medial malleolus fracture | 3 (13.04) | 2 (8.70) | ||
| Plantar fasciitis | 1 (4.35) | 3 (13.04) | ||
| Pott's fracture | 8 (34.78) | 7 (30.43) | ||
| Removal of tibial plate and screws | 3 (13.04) | 3 (13.04) | ||
| Tibial plateau fracture | 3 (13.04) | 3 (13.04) |
Patients’ Characters and Duration and Type of Surgery Between the Studied Groups a
The time to first need for rescue analgesia was significantly longer in the PD group at 7.76 ± 0.79 hours compared to the B group at 4.48 ± 0.63 hours (P < 0.001) (Table 2).
| Variables | PD Group; (N = 23) | B Group; (N = 23) | t | P-Values |
|---|---|---|---|---|
| Onset of sensory block (min) | 6.39 ± 1.12 | 6.43 ± 1.99 | 0.127 | 0.899 |
| Onset of motor block (min) | 10.09 ± 2.23 | 9.96 ± 2.33 | 0.194 | 0.847 |
| Time of regression of sensory block to L5 (min) | 146.74 ± 15.35 | 188.44 ± 6.84 | 11.902 | 0.0001 |
| Time of regression of motor block to Bromage 0 (min) | 119.56 ± 14.13 | 168.04 ± 5.25 | 15.418 | 0.0001 |
| Time of the first need for rescue analgesia (h) | 7.76 ± 0.79 | 4.48 ± 0.63 | 15.507 | 0.0001 |
The difference in the onset of sensory or motor blocks between the PD group (6.39±1.12 and 10.09 ± 2.23 minutes, respectively) and the B group (6.43 ± 1.99 and 9.96 ± 2.33 minutes, respectively) was not statistically significant. However, the time to regression of the sensory block was significantly shorter in the PD group compared to the B group (146.74 ± 15.35 minutes vs. 188.44 ± 6.84 minutes, respectively). The same significant difference was observed in motor block duration, with regression to Bromage score 0 taking 119.56 ± 14.13 minutes in the PD group and 168.04 ± 5.25 minutes in the B group (Table 2).
In terms of intraoperative hemodynamics, the incidence of hypotension was significantly lower in the PD group (2 cases, 8.70%) compared to the B group (8 cases, 34.78%). This was a significant difference, although the incidence of bradycardia was not significantly different between the groups (1 case in the PD group and 5 cases in the B group) (Table 3).
The incidence of adverse events varied, with shivering significantly lower in the PD group (1 case, 4.35%) than in the B group (9 cases, 39.13%). Pruritus occurred only in the PD group (2 cases, 8.69%), with no cases in the B group. The incidence of nausea, vomiting, respiratory depression, and sedation was comparable between the two groups (Table 4).
| Variables | PD Group; (N = 23) | B Group; (N = 23) | χ2 | P-Value |
|---|---|---|---|---|
| Shivering | 1 (4.35) | 9 (39.13) | 8.18 | 0.004 b |
| Nausea & Vomiting | 7 (30.43) | 6 (26.09) | 0.11 | 0.74 |
| Pruritus | 2 (8.70) | 0 (0) | f | 0.489 |
| Respiratory depression | 1 (4.3) | 0 (0) | f | 0.99 |
| Sedation | 1 (4.3) | 0 (0) | f | 0.99 |
Incidence of Perioperative Adverse Effects a
5. Discussion
This randomized comparative study evaluated the effectiveness of combining intrathecal pethidine and dexamethasone versus standard intrathecal bupivacaine for lower extremity orthopedic procedures. The findings demonstrated that the pethidine-dexamethasone combination provided superior spinal anesthesia, extended postoperative analgesia, minimal intraoperative hemodynamic fluctuations, and a reduced incidence of shivering compared to the bupivacaine group. However, the duration of the sensory and motor blocks was shorter in the pethidine-dexamethasone group than in the bupivacaine group.
Pethidine (meperidine) is unique in the anesthetic field, acting as both an opioid agonist on mu and kappa receptors and possessing local anesthetic properties similar to cocaine. Its analgesic potency is approximately eight times lower than morphine, with peak analgesia occurring within 30–50 minutes. Additionally, pethidine has anticholinergic properties, which reduce salivation and increase heart rate. In a 100 mg/mL solution, pethidine is hyperbaric, with a density of 1.0083 g/mL at 37°C (4, 14).
The study found that the onset of a complete motor block (Bromage score 4) was 10.09 ± 2.23 minutes in the pethidine-dexamethasone group, compared to 9.96 ± 2.33 minutes in the bupivacaine group. Consistent with our findings, previous studies have shown that 0.4 - 1 mg/kg of subarachnoid pethidine achieves effective motor and sensory block with improved postoperative analgesia in lower limb surgeries (15-18).
Pethidine is primarily metabolized in the liver, where it is demethylated to form normeperidine, an active metabolite with a half-life of approximately 15 - 30 hours in individuals with normal liver and kidney function. In patients with impaired renal or hepatic function or with repeated dosing, normeperidine can accumulate and may lead to seizures. Additionally, there is a hazardous interaction between pethidine and monoamine oxidase inhibitors (MAOIs), as their concurrent use can result in serotonergic overactivity (19).
In the current study, the time until the first need for rescue analgesia was significantly longer in the PD group (7.76 ± 0.79 hours) compared to the B group (4.48 ± 0.63 hours). This finding aligns with the study by Rezvani Habibabadi et al., which compared the effectiveness of 1 mg/kg meperidine to bupivacaine for spinal anesthesia in anorectal procedures and found that meperidine provided superior postoperative pain relief (20). Similarly, Fyrfiris et al. investigated the efficacy of a low dose (0.4 mg/kg) of intrathecal pethidine compared to ropivacaine with fentanyl for TURP surgeries. Their study concluded that 0.4 mg/kg subarachnoid pethidine provided adequate anesthesia with a longer interval before the first request for analgesia, consistent with our results (17).
Bayar et al. examined the effects of increasing doses of intrathecal pethidine (40, 50, 60, and 70 mg) for open prostatectomy under spinal-epidural anesthesia. They observed that doses of 60 and 70 mg of subarachnoid pethidine achieved complete motor block and prolonged analgesia, supporting our findings (16).
The use of dexamethasone, either intravenously or perineurally, alongside local anesthetics has been shown to improve both the duration and quality of analgesia. The enhanced postoperative analgesia associated with dexamethasone may be due to its anti-inflammatory properties and its ability to suppress nociceptive transmission in C-fibers (8, 11).
The study by Udonquak et al. compared the recovery profiles of subarachnoid pethidine and bupivacaine, reporting that intrathecal pethidine at 1 mg/kg resulted in a faster recovery, with S2 dermatomal sensation returning after 94.62 minutes compared to 205.96 minutes in the bupivacaine group. In the present study, the duration of the sensory block in the pethidine group was extended to 146.74 minutes, likely due to the addition of intrathecal dexamethasone. Nonetheless, consistent with previous studies, the sensory and motor block durations remained longer in the bupivacaine group (21).
This study also found that shivering and hypotension were less frequent in the group receiving pethidine combined with dexamethasone. Similarly, a meta-analysis by Afzal et al. concluded that intrathecal pethidine effectively reduced spinal anesthesia-induced shivering during elective cesarean sections without significantly affecting maternal blood pressure, though it did increase the risk of pruritus and vomiting (22).
Pethidine's effectiveness in reducing shivering is thought to be linked to its activity on kappa opioid receptors (19). The anti-shivering mechanism of dexamethasone may involve its inhibition of vasoconstrictive and pyrogenic cytokines (10).
A study conducted by Moeen, S and Moeen, A compared the effects of adding either 8 mg of intrathecal dexamethasone or 0.2 mg/kg of intrathecal pethidine to bupivacaine in spinal anesthesia. Both additives effectively reduced post-spinal shivering during TURP operations, consistent with our findings, though our study used a smaller dose of intrathecal dexamethasone at 4 mg (12). Furthermore, our results align with those of Tkachenko and Pyasetska, who reported that adding 4 mg of intrathecal dexamethasone as an adjuvant for spinal anesthesia in elective cesarean sections reduced complications such as shivering, nausea, vomiting, and arterial hypotension (9). However, in contrast to our findings, Zangoue et al. observed that intrathecal pethidine at a higher dose of 100 mg caused a more significant decline in blood pressure and heart rate during elective cesarean sections (23).
Ashoor et al. investigated the effects of a single preoperative intravenous dose of 8 mg dexamethasone on spinal-induced hypotension in elderly patients undergoing orthopedic surgery. The study found that post-spinal anesthesia hypotension, vomiting, and shivering were significantly reduced in the dexamethasone group compared to the placebo group, consistent with our results. However, our study administered a lower dose of dexamethasone (4 mg) via the intrathecal route (24).
5.1. Limitations
In the current study, the duration of surgeries did not exceed 90 minutes, limiting our assessment of the efficacy of intrathecal pethidine combined with dexamethasone in longer procedures. The relatively short duration of the sensory and motor blocks poses a challenge to the use of intrathecal pethidine alone for surgeries lasting more than 2 hours. Further studies are recommended to establish a consensus and clarify the effects and optimal use of intrathecal pethidine for extended procedures.
5.2. Conclusions
The combination of intrathecal 1 mg/kg pethidine with 4 mg dexamethasone provided superior spinal anesthesia, extended postoperative analgesia, minimized intraoperative hemodynamic disturbances, and reduced the incidence of shivering compared to the bupivacaine group. This approach may be particularly valuable for patients with hypersensitivity to ester or amide local anesthetics, offering a cost-effective alternative to standard anesthetics.