1. Background
Perioperative pain associated with major surgeries plays a significant role in the development of chronic pain in the pediatric patient population, with an incidence of chronic postoperative pain reaching up to 20% (1). Open surgeries are still widely adopted for the management of renal and suprarenal tumors requiring partial or total nephrectomy, which increases the risk of perioperative pain (2). Due to the subjective nature of pain, many pediatric patients suffer from underdiagnosis and undertreatment of their postoperative pain (3). Improper pain control can result in deleterious postoperative morbidities (4). Various analgesic techniques are employed to achieve adequate analgesia during pediatric surgeries, ranging from simple nonsteroidal anti-inflammatory drugs to opioids, regional fascial blocks, and neuraxial analgesia (5).
Since its introduction into practice by Campbell in 1933 (6), caudal analgesia has become one of the most commonly used blocks in various pediatric surgical interventions (7). Although the use of caudal block carries a low risk of complications, it can occasionally result in undesirable side effects such as lower limb sensory impairment, transient lower limb weakness, or bladder dysfunction, in addition to unintentional dural or rectal puncture (8). Since the introduction of the Erector Spinae Plane Block (ESPB) by Forero et al. in 2016 (9), it has gained increased popularity in perioperative pain management across diverse age groups. The ESPB is an easy, safe, and effective technique used for various pediatric surgical procedures (10). The craniocaudal spread of local anesthetic injected in lower thoracic ESPB provides visceral and somatic analgesia for abdominal surgeries (11).
In the pediatric population, regional blocks — whether central, peripheral, or locoregional — are performed under general anesthesia or sedation, making the use of ultrasound guidance crucial for precise injection and reducing inadvertent injections and subsequent unwanted complications (12).
2. Objectives
The current study aims to investigate the perioperative analgesic efficacy and safety of ultrasound-guided ESPB versus ultrasound-guided caudal block in pediatric cancer patients undergoing surgical resection for renal and suprarenal tumors.
3. Methods
This double-blinded (patients’ guardian and outcome assessor) randomized controlled clinical trial was conducted at the National Cancer Institute, Cairo University, Egypt, from December 2021 to July 2024. The study was approved by the institutional review board committee (IRB number: AP2109-30109) and was registered prospectively at ClinicalTrials.gov (NCT05153720). Informed consent was obtained from the patients’ guardians prior to participation.
3.1. Sample Size Calculation
In the current study, the authors compared the ESPB with caudal analgesia, the latter being the most commonly used block in various pediatric surgical interventions, serving as an "active control". Therefore, we adopted the non-inferiority concept in our trial to determine if ESPB is not less effective than the conventional caudal block in reducing total morphine consumption. The sample size calculation was performed using PASS software (version 11.0; NCSS PASS, UT, USA). The primary outcome of this non-inferiority trial is postoperative morphine consumption in the first 24 hours. The sample size was based on the following considerations: A 95% confidence limit, 95% power of the study, a group ratio of 1:1, a standard deviation of postoperative morphine consumption in the first 24 hours of 0.049 mg according to a previous study (13), and a non-inferiority margin set to 0.04 mg. To account for possible attrition, 40 patients were recruited in each group.
3.2. Randomization and Concealment
Patients were randomly assigned to one of the two study groups using a computer-generated randomization sequence. Patient allocation was concealed through the use of a closed-envelope technique. An investigator not involved in patient care handed the envelope to the anesthetist responsible for administering the block. The block was performed by an expert anesthesiologist who was not involved in data collection.
3.3. Eligibility Criteria
The eligibility of patients was assessed in the preoperative anesthesia clinic. Both sexes, aged 1 to 8 years, ASA II and III patients diagnosed with renal and nonfunctioning suprarenal tumors were recruited. Patients with functioning tumors, low platelet count, and impaired renal and/or kidney function were excluded from the study. Additionally, patients who experienced massive blood loss during the study were excluded.
3.4. Outcome Measures
The primary outcome measure was the total postoperative morphine consumption in the first 24 hours. Secondary outcomes included rescue intraoperative fentanyl consumption, intraoperative and postoperative hemodynamics, time to first postoperative analgesia, postoperative FLACC (Face, Legs, Activity, Cry, Consolability) pain scores, and any complications such as a failed block, hypotension, bradycardia, vomiting, and local anesthetic systemic toxicity. The FLACC score is assessed based on five parameters, each evaluated on a 3-point scale: Zero, 1, and 2. The total score ranges from 0 to 10, with 0 indicating no pain, 1 - 3 indicating mild pain, 4 - 7 indicating moderate pain, and 8 - 10 indicating severe pain.
3.5. General Anesthesia
All patients were reassessed in the holding area. Premedication was administered with oral midazolam (0.5 mg/kg). Conventional general anesthesia was induced with intravenous (IV) fentanyl (2 μg/kg), propofol (2 mg/kg), and IV rocuronium (0.5 mg/kg). Anesthesia was maintained with sevoflurane at 2%. Mechanical ventilation was adjusted to maintain end-tidal CO2 (ETCO2) between 35 and 40 mmHg. Intraoperatively, if there was an increase in heart rate (HR) or mean arterial pressure (MAP) above baseline values by 20%, and after excluding inadequate anesthesia, fentanyl (0.5 μg/kg) was administered and documented.
3.6. Caudal Technique
Anesthetized patients were placed in the lateral position. After aseptic preparation and towel draping, a high-frequency ultrasound linear probe (6 - 13 MHz) of the SonoSite M-Turbo (FUGIFILM, USA) was applied transversely to the sacral area along an imaginary line transecting the two sacral cornua. The intended block area was visualized as two hyperechoic densities representing the sacral cornua, with an underlying hypoechoic area representing the injection plane between two hyperechoic lines that represent the sacrococcygeal ligament and the deeper bony sacrum. A 22-gauge needle was advanced using an out-of-plane technique between the sacral cornua to penetrate the sacrococcygeal ligament. The probe was then placed in a linear orientation to visualize the needle shaft, and 1 mL of normal saline was injected into the sacral epidural space for hydrodissection and confirmation of proper needle position. After negative aspiration of blood or cerebrospinal fluid (CSF), 1.25 mL/kg of 0.125% bupivacaine, with a maximum dose of 2.5 mg/kg, was injected carefully.
3.7. Erector Spinae Plane Block Technique
Patients were placed in the lateral position. After aseptic preparation and towel draping, the seventh thoracic spinous process was identified by counting down from the seventh cervical spine, recognized as the largest bony prominence at the back of the neck. Using a linear high-frequency probe (6 - 13 MHz) of the SonoSite M-Turbo (FUGIFILM, USA), the transverse process of T7 was visualized 1 - 2 cm lateral to the seventh thoracic spinous process. The ultrasound probe was placed longitudinally to identify the entry point. A 22-gauge needle was advanced in a craniocaudal trajectory using an in-plane technique to penetrate the trapezius muscle and the erector spinae muscles overlying the hyperechoic transverse process. Correct needle position was confirmed through hydrodissection, which elevated the erector spinae muscles from the transverse process. After negative aspiration of blood or CSF, 0.5 mL/kg of 0.25% bupivacaine was injected carefully. The maximum allowed dose for injection was set at 2.5 mg/kg.
3.8. Post-intervention
At the end of the surgery, patients were extubated and admitted under observation to the post-anesthesia care unit (PACU) for monitoring of HR, MAP, and FLACC scores at 2, 4, 8, 12, 16, 20, and 24 hours. Routine postoperative analgesia with paracetamol 15 mg/kg was administered every 8 hours. In cases where FLACC scores were ≥ 4, IV morphine at a dose of 0.05 mg/kg was administered and recorded. Any complications related to the block, such as hematoma or suspected local anesthetic toxicity, were managed and reported. The incidence of nausea and vomiting was also reported and managed.
3.9. Statistical Analysis
Data were analyzed using SPSS version 26 (IBM Inc., Chicago, IL, USA). The normality of data distribution was assessed using the Shapiro-Wilk test and histograms. Mean and standard deviation (SD) were used to represent quantitative parametric data, and comparisons between two groups were performed using the unpaired Student's t-test. Median and interquartile range (IQR) were used to represent quantitative non-parametric data and were analyzed using the Mann-Whitney test. Frequency and percentage (%) were used to represent qualitative variables, which were analyzed using the chi-square test or Fisher's exact test when appropriate. A P-value < 0.05 was considered statistically significant.
4. Results
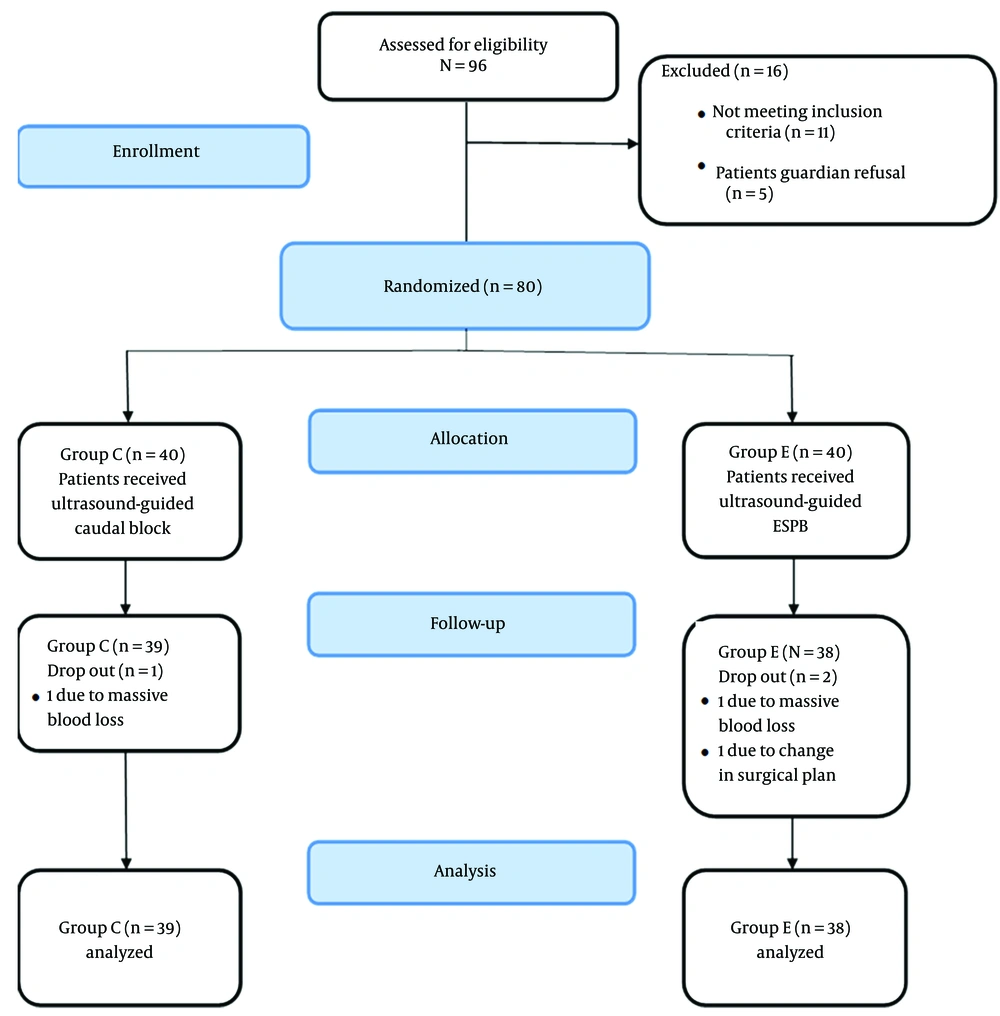
Ninety-six patients were initially recruited and assessed for eligibility. Eighty patients were randomly assigned to one of the two study groups. Two patients were excluded from the ESPB group: One due to massive blood loss and the other due to a change in the surgical decision. One patient was excluded from the caudal group due to massive blood loss (Figure 1). Both groups had comparable demographic data, duration of intervention, and type and duration of surgery (Table 1).
| Variables | Group C (n = 39) | Group E (n = 38) | P-Value |
|---|---|---|---|
| Age (mo) | 32.1 ± 16.4 | 37.1 ± 15.4 | 0.174 |
| Gender | 0.701 | ||
| Male | 16 (41.1) | 14 (36.8) | |
| Female | 23 (58.9) | 24 (63.2) | |
| ASA | |||
| II | 34 (87.2) | 31 (81.6) | |
| III | 5 (12.8) | 7 (18.4) | |
| Weight (kg) | 13.1 ± 2.8 | 14.2 ± 2.3 | 0.070 |
| Height (cm) | 90.0 ± 11.1 | 94.0 ± 9.2 | 0.083 |
| Type of surgery | 0.813 | ||
| Nephrectomy | 29 (74.4) | 27 (71.1) | |
| Partial nephrectomy | 5 (12.8) | 5 (13.1) | |
| Adrenalectomy | 5 (12.8) | 6 (15.8) | |
| Duration of intervention (min) | 16.9 ± 1.6 | 16.9 ± 1.7 | 0.806 |
| Duration of surgery (min) | 178 ± 40 | 181 ± 36 | 0.743 |
a Values are expressed as mean ± SD or No. (%).
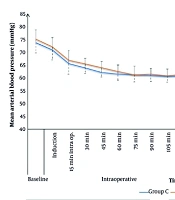
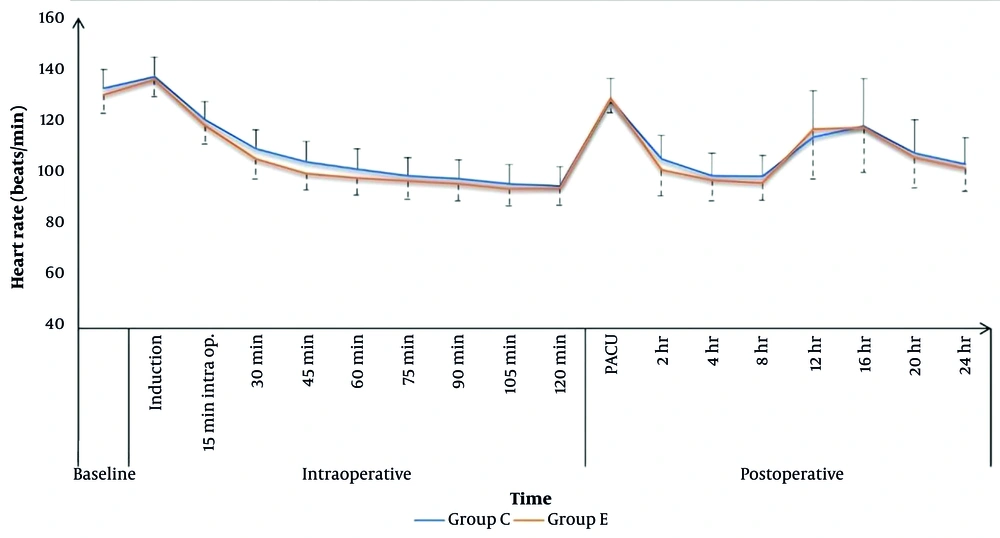
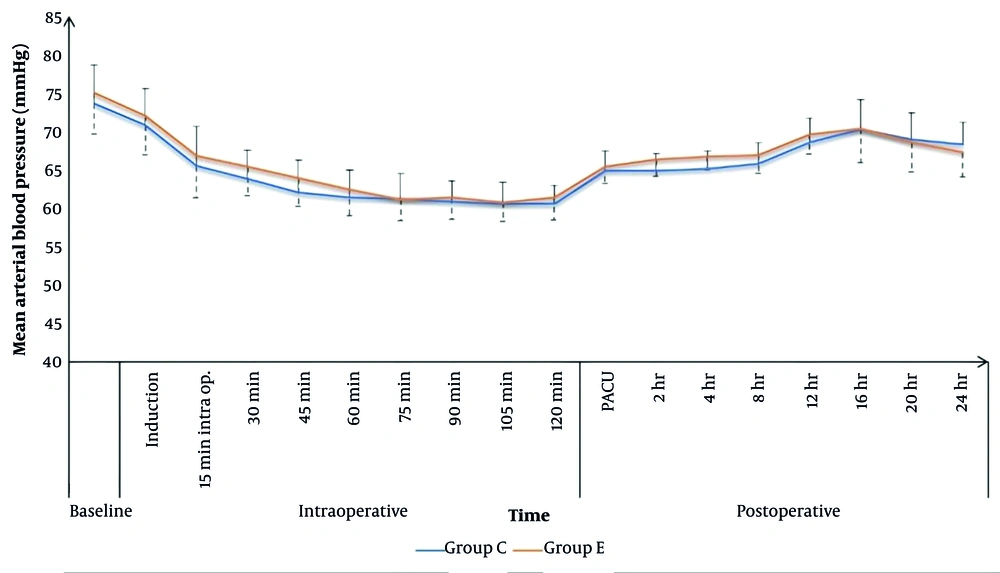
Heart rate values were comparable between the two groups throughout the intraoperative period, except at 30, 45, and 60 minutes, where the caudal group had higher HR values (109 ± 7, 104 ± 8, and 101 ± 8 bpm) compared to the ESPB group (105 ± 7, 99 ± 6, and 97 ± 6 bpm), respectively, with P < 0.05 (Figure 2). Intraoperative MAP values were comparable for both groups, except at 45 minutes, where the caudal group (62 ± 4 mmHg) showed statistically lower values compared to the ESPB group (64 ± 4 mmHg), P = 0.039 (Figure 3).
In the PACU and for the first 24 hours postoperatively, HR values were comparable for the two study groups, except that the caudal group showed significantly higher HR values after 2 and 8 hours (106 ± 9 and 99 ± 8 bpm) compared to the ESPB group (100 ± 9 and 95 ± 6 bpm), respectively, with P < 0.05. Postoperative MAP in the PACU and for the first 24 hours postoperatively was comparable for the two groups, except after 2 and 4 hours, the caudal group had significantly lower MAP values (65 ± 2 and 65 ± 2 mmHg) compared to the ESPB group (66 ± 2 and 67 ± 2 mmHg), respectively, with P < 0.05 (Figures 2 and 3).
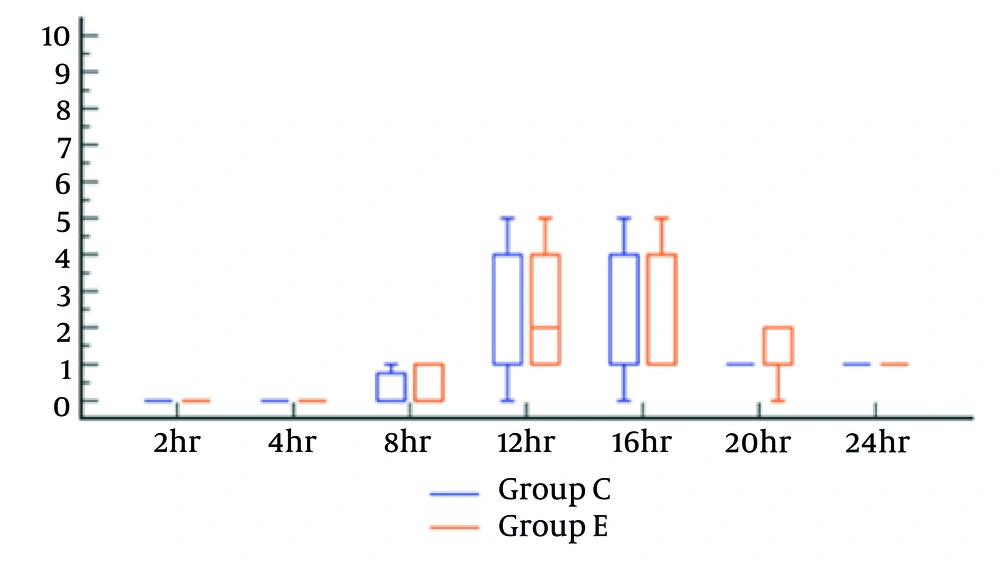
No patients in either group required intraoperative fentanyl (Table 2). The postoperative FLACC scores showed no significant difference between the study groups (Figure 4). The time to first postoperative rescue analgesic was comparable between the caudal and ESPB groups (15.0 ± 3.0 and 14.4 ± 2.3 hours, respectively). There were no reported complications for either group. Total 24-hour postoperative morphine consumption was significantly lower in the caudal group (1.21 ± 0.66 mg) compared to the ESPB group (1.61 ± 0.34 mg), P = 0.002 (Table 2).
| Variables | Group C (n = 39) | Group E (n = 38) | P-Value |
|---|---|---|---|
| Patients required intraoperative fentanyl | 0 (0) | 0 (0) | - |
| Total 24 h postoperative morphine consumption | 1.21 ± 0.66 | 1.61 ± 0.33 | 0.002 b |
| Time to first analgesic | 15.0 ± 3.0 | 14.4 ± 2.3 | 0.316 |
a Values are expressed as No. (%) or mean ± SD.
b Significant as P-value ≤ 0.05.
The non-inferiority analysis for total 24-hour postoperative morphine consumption showed a mean of 1.61 mg with a 95% CI (1.50 - 1.71) for the ESPB group versus 1.21 mg with a 95% CI (1.04 - 1.45) for the caudal group. The mean difference in morphine consumption was 0.36, with the 95% confidence interval of the difference (0.13 - 0.59). We cannot declare the non-inferiority of ESPB compared to the caudal block, as the predetermined margin of inferiority (0.04 mg) is below the lower limit of the CI of the difference between groups.
5. Discussion
Various analgesic modalities are employed for pediatric perioperative pain control. Combined general-regional anesthesia is a common practice for pediatric surgeries. The introduction of ultrasound guidance for regional interventions has demonstrated several benefits, including increased safety and improved outcomes (12, 14). This study compared the efficacy and safety of ultrasound-guided (USG) caudal block with 1.25 mL/kg of 0.125% bupivacaine versus USG ESPB with 0.5 mL/kg of 0.25% bupivacaine in pediatric cancer patients undergoing renal and suprarenal resection surgeries. The volumes and concentrations were chosen to mitigate any potential local anesthetic toxicity associated with higher volumes or concentrations.
Caudal analgesia has been one of the most commonly used blocks in pediatric surgeries for decades, while ESPB is a relatively recent technique that has rapidly gained popularity in both adults and pediatrics. The current study demonstrated more hemodynamic stability with ESPB, with comparable postoperative pain scores between the two interventions, as well as a comparable time to receive the first postoperative analgesia (15.0 ± 3.0 and 14.4 ± 2.3 hours for the caudal and ESPB groups, respectively). However, morphine consumption in the first 24 hours was higher for the ESPB group (1.61 ± 0.33 mg) compared to the caudal group (1.21 ± 0.66 mg). Thus, we could not declare the non-inferiority of ESPB compared to the caudal block.
In a study conducted by Elshazly et al. comparing the analgesic effect of lumbar USG ESPB to caudal analgesia in pediatric patients undergoing hip and proximal femur surgeries, they concluded that ESPB did not provide a superior analgesic effect compared to caudal analgesia. They reported a prolonged duration before the first postoperative analgesic administration for the caudal group, while our study reported lower total postoperative morphine consumption for the ESPB group compared to the caudal group (15).
In a study by Abotaleb et al. comparing caudal block to ESPB for pediatric lower limb surgeries, their results showed a superior analgesic effect of ESPB over the caudal block. These results were explained by lower pain scores, extended analgesia, and less postoperative analgesic consumption for the ESPB group. In accordance with our findings, they reported that ESPB showed more stable hemodynamics compared to the caudal block (14).
However, we observed different results regarding the analgesic effects, which might be attributed to the different volumes used in each study. In our study, we injected 0.5 mL/kg of local anesthetic at the level of T7, which in a child with an average weight of 14 kg resulted in a total volume of approximately 7 mL of injectate. In contrast, the other study injected 20 mL beneath the erector spinae muscles at the level of L1-L4.
In 2024, Pandey et al. published a study comparing the analgesic efficacy of USG ESPB with 0.5 mL/kg of 0.25% bupivacaine versus USG caudal block with 1 mL/kg of 0.25% bupivacaine in pediatric patients undergoing abdominal surgeries. They reported that ESPB, as part of multimodal analgesia, can be considered safe in pediatric patients undergoing abdominal surgeries but showed an inferior analgesic profile compared to caudal analgesia. They explained their results by the higher FLACC scores and a greater percentage of patients requiring analgesia in the ESPB group, with a shorter duration for the first postoperative analgesic request (16).
In another study conducted by Abdelrazik et al., they concluded the superiority of ESPB with 0.4 mL/kg of 0.25% bupivacaine compared to caudal block with 2.5 mg/kg of 0.25% bupivacaine in patients aged 2 to 6 years who underwent unilateral lower abdominal surgeries. They reported lower pain scores for the ESPB, with a longer duration of postoperative analgesia and lower postoperative analgesic requirements (17).
Mostafa et al. assessed the efficacy of bilateral ESPB with 0.3 mL/kg of 0.25% bupivacaine for perioperative pain control in pediatric patients undergoing midline incision. They reported lower intraoperative and postoperative analgesic requirements with better pain scores in the first postoperative 8 hours (18).
The results of Guan et al. on pediatric patients undergoing unilateral hernia repair showed a superior analgesic profile of ESPB with an injection of 0.5 mL/kg of 0.2% ropivacaine compared to caudal block with 1 mL/kg of 0.2% ropivacaine. They reported a longer duration to receive postoperative analgesia for the ESPB group compared to the caudal group, with lower postoperative FLACC scores. The differences between their study and ours are mainly in the type of surgery and the volume injected for the caudal block (19).
In our study, although we could not prove the non-inferiority of ESPB compared to the caudal block, we support that ESPB can be used as a reliable, effective alternative to caudal analgesia. Erector spinae plane block has the advantage of comparable pain reduction and duration of postoperative analgesia. Additionally, the risk of inadvertent intravascular injection is considered very low with ESPB compared to the caudal block due to the anatomical differences between the targeted areas of block injection, which increases the reliability and safety of ESPB as an alternative to the caudal block.
5.1. Conclusions
When compared to USG caudal analgesia, USG ESPB showed more hemodynamic stability but had increased postoperative morphine consumption with a comparable first-time to postoperative analgesic requirement and postoperative FLACC scores. Therefore, we could not declare the non-inferiority of ESPB compared to the caudal block.
5.2. Limitation
This trial's limitations include its single-center design and the limited number of patients. We believe that further studies involving a larger patient population, a multicentric approach, and different types of surgeries could help in evaluating the efficacy and safety of USG ESPB in the pediatric patient population.