1. Background
Opioid medication administration is a common method for relieving pain from surgical trauma. However, excessive doses of opioids during and after surgery can increase the risk of various side effects, including respiratory depression, drowsiness, nausea, emesis, pruritus, difficulty urinating, and ileus (1). In craniofacial surgeries involving maxillomandibular fixation, these adverse effects are distressing for patients and, in severe cases, may lead to life-threatening complications. The most serious side effects are ventilatory depression and vomiting, particularly in the early postoperative period. To mitigate these negative effects, various methods have been recommended. A long-acting local anesthetic nerve block is one method suggested for this specific area (2, 3). Bupivacaine is an effective long-acting local anesthetic used in maxillofacial surgery. The inferior alveolar nerve block (IANB) can be used to suppress hemimandible sensory function. It can provide adequate anesthetic and pain relief for one side of the mandibular teeth and gingival mucosa, the body and inferior ramus of the mandible, the anterior two-thirds of the tongue, and the floor of the oral cavity (4, 5). The primary method for IANB is the direct procedure, which involves placing the needle tip into the pterygomandibular raphe by traversing the buccinator muscle. The goal in this area is to deliver the local anesthetic solution near the inferior alveolar nerve (IAN) before it enters the mandibular foramen (6). Reports indicate that between 10% and 39% of IANBs fail. This high failure rate can be attributed to poor anesthetic technique, clinical derangements, or anatomical changes in tissues surrounding the IAN (7, 8).
2. Objectives
This study aimed to evaluate the efficacy of two methods for managing pain during surgery for mandibular fractures: Conventional intravenous analgesia and a pre-emptive IANB. The primary objective was to assess the intensity and duration of the analgesic effect of the IANB. This was achieved by measuring the time to the first dose and total doses of fentanyl rescue analgesia intraoperatively, as well as the time to the first dose and total doses of pethidine as rescue analgesia postoperatively, using the Visual Analog Scale (VAS) in both groups.
3. Methods
We conducted this prospective randomized experiment at Ain Shams University Hospitals from June 2023 to June 2024, with approval from the Clinical Trial Registry (NCT06167187) and the Research Ethics Committee (ERC) at the Faculty of Medicine, Ain Shams University. The sample size was calculated using the PASS 15 program, setting power at 90% and an alpha error of 0.05. According to Mesgarzadeh et al., 2014, the expected rate of need for analgesia was 20% in the IANB group compared to 70% in the control group (9). A sample size of 23 patients per group was sufficient to detect the difference between the two groups.
With written and informed consent, we included America Association of Anesthesia (ASA) I and II patients aged 18 to 65 years. These patients were scheduled for surgery for solitary mandibular fractures under general anesthesia. Exclusion criteria included refusal to participate, allergy to study medications, pregnancy, mental illness, coagulopathy, local infections, history of addiction, obstructive sleep apnea, and the need for postoperative mechanical ventilation and intensive care unit admission.
In the preoperative room, all patients received information regarding the analgesic regimen and were instructed on using the VAS to communicate their pain intensity. The VAS is a 10-centimeter-long unlabeled line where 0 denotes no pain, 1 - 3 indicates mild pain, 4 - 6 represents moderate pain, and 7 - 10 signifies severe pain. Upon the patient's arrival in the operating room, initial measurements were taken, including systolic and diastolic blood pressures, mean pulse rates, and oxygen saturation levels.
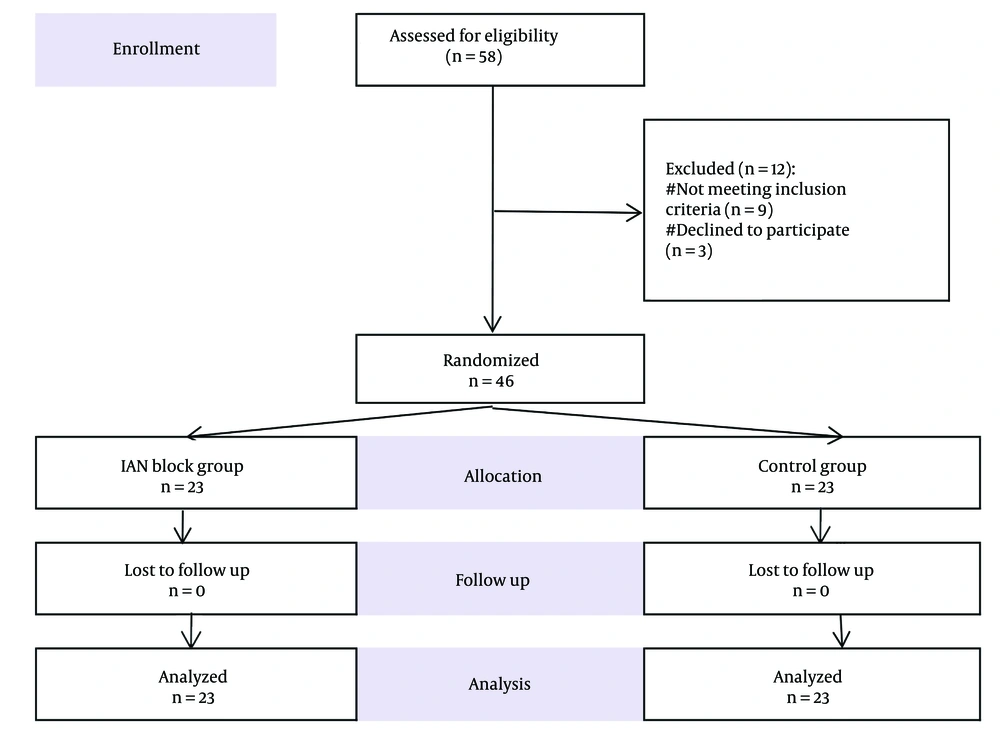
According to the CONSORT statement, a total of 58 patients were assessed for eligibility. Twelve patients were excluded from the study due to coagulopathy (n = 2), a history of addiction (n = 3), ASA physical status class IV (n = 4), and refusal to participate (n = 3). A total of 46 patients were included in this experiment and randomized into two equal groups (Figure 1). An impartial therapist, not involved in the study, prepared and administered the IANB after selecting an opaque sealed envelope from a box based on a computer-generated numerical sequence. Both the patients and the second investigator, who collected the data and administered rescue analgesics if necessary, were blinded to the group assignments.
The group assignments were indicated on the letters included in each envelope: (A) Patients in group A (IANB group) received bilateral IANB after endotracheal intubation and before surgical positioning and incision; (B) as a control, patients in group B received intravenous multimodal analgesia according to the established protocol instead of the block treatment.
Patients were moved to the operating theater, where monitoring for ECG, non-invasive blood pressure (NIBP), SpO2, temperature, and end-tidal CO2 (EtCO2) was initiated, and an intravenous line was established. All patients received general anesthesia through intravenous administration of fentanyl (1 µg/kg), propofol (2 mg/kg), and atracurium (0.5 mg/kg). Endotracheal intubation was conducted using a suitably sized cuffed endotracheal tube, which was secured following the verification of bilateral equal air entry through auscultation and confirmation with EtCO2. Mechanical ventilation was maintained to ensure end-expiratory CO2 levels between 34 and 45 mmHg, as monitored by capnography.
Patients were administered isoflurane at concentrations of 1 - 2 vol% in a mixture of 50% oxygen and 50% air. Atracurium was given in increments of 0.1 mg/kg every 30 minutes or as needed. Granisetron was administered at a dosage of 3 mg to prevent postoperative nausea and vomiting. Ringer's solution was administered at a rate of 4 mL/kg/h throughout the surgical procedure. Intraoperative administration of fentanyl at a dosage of 1 - 2 µg/kg was implemented if the heart rate (HR), blood pressure, or both exceeded a 20% increase from baseline levels. The first time to need intraoperative fentanyl and the total amount of additional intraoperative fentanyl were recorded. Vital signs, including HR and mean arterial pressure (MAP), were recorded every 10 minutes intraoperatively until the end of surgery. Approximately 30 minutes prior to the conclusion of the surgical procedure, all patients were administered 1 g of intravenous paracetamol.
The IANB was bilaterally administered after completely drying the pterygomandibular triangle with gauze. This fatty area is laterally bounded by the coronoid notch and medially by the pterygomandibular raphe, a visible tendinous line formed by the junction of the buccinator and superior pharyngeal constrictor muscles. This procedure followed endotracheal intubation and preceded surgical positioning and incision. When necessary, suction was used to keep the area dry. The tip of the thumb or forefinger was placed into the coronoid notch located posterior to the molars. Subsequently, the cheek was retracted to expose the pterygomandibular triangle. A 25-gauge needle, 3 cm long, was used for nerve blocks, with one needle on each side.
Place the needle tip in the pterygomandibular triangle, with the bevel oriented towards the ramus. Position the syringe's barrel above the contralateral lower first and second premolars, ensuring the needle's side rests against the lateral edge of the pterygomandibular raphe. Gently insert the needle tip into the mucosa until it reaches the ramus, typically after 2 to 2.5 cm of insertion, and then retract the needle by 1 mm from the bone. Withdraw the needle 2 to 3 mm after aspiration to verify the absence of intravascular placement; if aspiration suggests intravascular implantation, repeat aspiration prior to injection. Administer a gradual injection of 2 to 4 mL of 0.5% bupivacaine anesthetic to each side, followed by massage of the injection sites.
Following our hospital's standard postoperative procedures, patients were extubated upon completion of the procedure and, after regaining consciousness, were transferred to the recovery room for monitoring. The VAS was used to assess and manage postoperative pain upon arrival to the post-anesthesia care unit (PACU) and after 30 minutes, then in the surgical ward every 2 hours during the first 6 hours and every 6 hours for 24 hours postoperatively. If VAS ≥ 3 postoperatively, 25 mg of pethidine was administered intravenously as a rescue analgesic (not exceeding 150 mg/day). Therefore, in both groups, intravenous pethidine was administered for immediate pain relief, while intravenous paracetamol was given every 6 hours and 30 mg ketorolac every 8 hours for prolonged pain management, in accordance with our hospital protocol for rescue analgesia.
3.1. Main Outcome Measures
The intensity and duration of the analgesic effect of the IANB were evaluated through the following primary outcomes: (A) The time to the first dose and total doses of fentanyl rescue analgesia administered intraoperatively; (B) the time to the first dose and total doses of pethidine used as rescue analgesia postoperatively. The secondary outcome included any adverse effects related to the IANB, such as local anesthesia toxicity, respiratory depression, hematoma, and infection, were noted.
3.2. Statistics
The data were encoded, organized, and statistically analyzed using IBM SPSS statistics software version 28.0 (IBM Corp., Chicago, IL, USA, 2021). Quantitative data were assessed for normality using the Shapiro-Wilk test and described as mean ± standard deviation (SD) with the minimum and maximum values of the range. These data were subsequently analyzed using an independent t-test. Qualitative data were defined by numerical values and percentages and examined using the chi-square test and Fisher’s exact test. The log-rank test was used to compare the frequencies of pethidine requests. The significance threshold was set at a P-value of ≤ 0.050; findings exceeding this threshold were considered non-significant.
4. Results
Table 1 indicates that no statistically significant differences were observed among the studied groups regarding age, gender, Body Mass Index (BMI), ASA grade, and operation duration.
| Variables | IANB Group (Total = 23) | Control Group (Total = 23) | P-Value |
|---|---|---|---|
| Age (y) | 26.7 ± 2.5 (22.0 - 31.0) | 25.8 ± 2.3 (22.0 - 31.0) | 0.252 b |
| Gender | 0.760 c | ||
| Male | 14 (60.9) | 15 (65.2) | |
| Female | 9 (39.1) | 8 (34.8) | |
| BMI (kg/m2) | 25.9 ± 2.5 (19.5 - 29.5) | 26.2 ± 2.4 (22.3 - 31.4) | 0.750 b |
| ASA | 0.999 d | ||
| I | 20 (87.0) | 19 (82.6) | |
| II | 3 (13.0) | 4 (17.4) | |
| Operation duration (min) | 102.7 ± 6.9 (90.0 - 117.0) | 104.3 ± 6.6 (96.0 - 119.0) | 0.424 b |
Abbreviations: BMI, Body Mass Index; ASA, America Association of Anesthesia; IANB, inferior alveolar nerve block.
a Values are expressed as mean ± SD (range) or No. (%).
b Independent t-test.
c Chi-square test.
d Fisher’s exact test.
Table 2 indicates that the requirement for fentanyl was markedly decreased in the IANB group. No statistically significant difference was found between the groups regarding the time taken for fentanyl administration. However, the dosage of intraoperative fentanyl was significantly lower in the IANB group.
| Variables | IANB Group (Total = 23) | Control Group (Total = 23) | P-Value | Relative Effect b Relative Risk |
|---|---|---|---|---|
| Fentanyl requirement (No. of patients) | 5 (21.7) | 17 (73.9) | < 0.001 c, d | - |
| The total number of patients who required fentanyl | 5 | 17 | ||
| Time to first dose analgesia (min) | 38.4 ± 1.7 (37.0 - 41.0) | 37.8 ± 1.2 (36.0 - 40.0) | 0.393 e | 0.6 ± 0.7 (-0.8, 2.0) f |
| Dose (mcg) | 55.0 ± 20.9 (25.0 - 75.0) | 83.8 ± 17.5 (50.0 - 100.0) | 0.006 d, e | -28.8 ± 9.3 (-48.2, -9.4) f |
Abbreviations: SE, standard error; CI, confidence interval; IANB, inferior alveolar nerve block.
a Values are expressed as mean ± SD (range) or No. (%).
b Relative effect: Effect in IANB relative to that in the control group.
c Chi-square test.
d P < 0.05 was considered statistically significant.
e Independent t-test.
f Values are expressed as mean ± SE (95% CI).
4.1. Intraoperative Hemodynamics Between the Studied Groups
Our study assessed the hemodynamic parameters, specifically HR and MAP, intraoperatively. The IANB group showed a lower trend throughout the surgery, with significantly lower values at minute 30. The mean ± SD of HR was 68.2 ± 4.7 in the IANB group compared to 71.3 ± 3.3 in the control group (P = 0.011). Similarly, MAP was 76.1 ± 5.3 in the block group versus 79.9 ± 3.6 in the control group (P = 0.007). At minute 40 after the beginning of the surgery, the HR was 68.0 ± 4.9 in the block group versus 71.7 ± 3.5 in the control group (P = 0.005), and the MAP was 76.3 ± 5.5 in the block group compared to 80.2 ± 4.3 in the control group (P = 0.011).
Table 3 shows that the postoperative pain score gradually increased in both study groups, peaking at hour 6 in the IAN block group and hour 2 in the control group. The postoperative pain score remained lower in the IAN block group from hour 1 to hour 24 postoperatively, with statistically significant differences observed from hour 1 to hour 6.
| Postoperative Time | IANB Group (Total = 23) | Control Group (Total = 23) | P-Value b | Relative Effect c Cohen’s d (95% CI) |
|---|---|---|---|---|
| Hour-0 | 0.0 ± 0.0 (0.0 - 0.0) | 0.0 ± 0.0 (0.0 - 0.0) | NA | NA |
| Hour-0.5 | 0.0 ± 0.0 (0.0 - 0.0) | 0.0 ± 0.0 (0.0 - 0.0) | NA | NA |
| Hour-1 | 0.1 ± 0.3 (0.0 - 1.0) | 0.8 ± 0.4 (0.0 - 1.0) | < 0.001 d | -1.9 (-2.6, -1.2) |
| Hour-2 | 0.7 ± 0.5 (0.0 - 1.0) | 3.9 ± 0.8 (2.0 - 5.0) | < 0.001d | -4.7 (-5.8, -3.5) |
| Hour-4 | 1.7 ± 0.8 (1.0 - 3.0) | 3.7 ± 0.9 (2.0 - 5.0) | < 0.001 d | -2.3 (-3.0, -1.5) |
| Hour-6 | 2.9 ± 0.7 (2.0 - 4.0) | 3.4 ± 0.9 (2.0 - 5.0) | 0.038 d | -0.6 (-1.2, 0.0) |
| Hour-12 | 2.7 ± 0.7 (2.0 - 4.0) | 3.0 ± 0.5 (2.0 - 4.0) | 0.066 | -0.6 (-1.1, 0.0) |
| Hour-18 | 2.0 ± 0.6 (1.0 - 3.0) | 2.3 ± 0.4 (2.0 - 3.0) | 0.103 | -0.5 (-1.1, 0.1) |
| Hour-24 | 1.4 ± 0.5 (1.0 - 2.0) | 1.7 ± 0.5 (1.0 - 2.0) | 0.080 | -0.5 (-1.1, 0.1) |
Abbreviations: NA, not applicable; SE, standard error; CI, confidence interval; IANB, inferior alveolar nerve block.
a Values are expressed as mean ± SD (range).
b Independent t-test.
c Relative effect: Effect in IANB relative to that in the control group.
d P < 0.05 was considered statistically significant.
Table 4 demonstrates that pethidine requests were significantly lower in the IANB group. The time to the first postoperative pethidine request was substantially longer in the IANB group. Additionally, the total 24-hour postoperative dose of pethidine was considerably lower in the IANB group. Regarding complications related to the IANB, only two patients exhibited minor hematomas at the site of the local anesthetic injection, which resolved spontaneously.
| Variables | IANB Group (Total = 23) | Control Group (Total=23) | P-Value | Relative Effect bRelative Risk |
|---|---|---|---|---|
| Pethidine request (No. of patients) | 8 (34.8) | 23 (100) | < 0.001 c, d | NA |
| The total number of patients who required pethidine | 8 | 23 | ||
| Time to first dose rescue analgesia (h) | 5.6 ± 0.7 (4.6 - 6.5) | 3.1 ± 0.4 (2.4 - 3.9) | < 0.001 d, e | 2.5 ± 0.2 (2.1, 2.9) f |
| Total 24-hour dose (mg) | 65.6 ± 12.9 (50.0 - 75.0) | 97.8 ± 16.7 (75.0 - 125.0) | < 0.001 d, e | -32.2 ± 6.5 (-45.5, -18.9) f |
Abbreviations: NA, not applicable; SE, standard error; CI, confidence interval; IANB, inferior alveolar nerve block.
a Values are expressed as mean ± SD (range) or No. (%).
b Relative effect: Effect in IANB block relative to that in the control group.
c Chi-square test.
d P < 0.05 was considered statistically significant.
e Independent t-test.
f Values are expressed as mean ± SE (95% CI).
5. Discussion
In maxillofacial surgery involving mandibular fracture surgeries, a strong sympathetic reaction is frequently observed. Significant opioid dosages are typically necessary to manage this sympathetic response (10). It is recommended to employ alternative analgesic techniques that reduce the necessity for opioids and their associated adverse effects in the surgical management of mandibular fractures (11, 12). The lower lip, gingiva, and mandibular teeth can be numbed using an IANB. Lower jaw tooth extractions and other small mandibular procedures typically use the IANB as their nerve block treatment of choice (13).
This study assessed the efficacy of pre-emptive IANB versus conventional systemic intravenous analgesia in reducing perioperative pain associated with mandibular fracture surgeries. In this investigation, age, gender, BMI, ASA grade, and operation time exhibited no significant differences between the IANB and control groups. We measured the need for fentanyl during surgery between the IANB and control group. The results demonstrated that the IANB group required much less fentanyl, with no statistically significant difference in the time to the first intraoperative fentanyl dose between the groups; nevertheless, the intraoperative fentanyl dosage was markedly lower in the IANB group.
We evaluated the hemodynamic parameters, particularly HR and MAP, between the block and control groups during the intraoperative period. Both parameters exhibited a gradual increase in both study groups, peaking at minutes 30 and 40, with significantly lower values observed in the IANB group at those time points. Shetmahajan et al. obtained analogous results in their randomized controlled study, which investigated the analgesic effectiveness of IANB during maxillofacial cancer surgery under general anesthesia. They found that the IANB significantly reduced the sympathetic response and the requirement for intravenous fentanyl during mandibular resection in maxillofacial surgery (14).
Since both groups were recuperating from anesthesia, there was no difference between them when they arrived at the PACU. The IANB consumed considerably less pethidine than the control group. In the IANB, the time required to request rescue analgesia was also much longer, lasting between 4 and 6 hours, compared to less than 4 hours in the control group. Postoperative pain scores increased gradually in both study groups to reach their peak at hour 6 in the IANB group and hour 2 in the control group. Postoperative pain score was lower in the IANB group from hour 1 until hour 24 postoperatively, but the differences were statistically significant from hour 1 to hour 6.
This aligns with Khan et al.'s analysis of the efficacy of intravenous tramadol versus bupivacaine IANB for postoperative analgesia in fractures of the mandibular parasymphyseal region. Post-surgery, the bupivacaine IANB demonstrated superior efficacy over tramadol in alleviating somatic wound pain, while exhibiting minimal adverse effects. Consequently, following general anesthesia, the administration of bupivacaine IANB is advised as a secure and effective postoperative analgesic (15).
Mesgarzadeh et al. investigated the effects of bilateral mental nerve block with bupivacaine on postoperative pain management in mandibular parasymphysis fractures, revealing that this combination can be both safe and effective in diminishing the necessity for opioid analgesics and alleviating postoperative pain and discomfort in affected patients. Despite implementing the bilateral inferior nerve block instead of the bilateral mental nerve block, which may present additional challenges, the outcomes were analogous to those of our study (9). Only 2 of 23 patients exhibited hematoma at the local anesthetic injection site, which resolved spontaneously. Therefore, a bilateral IANB is a safe technique for mandibular fracture surgeries, as it diminishes pain intensity during the perioperative period, reduces total opioid consumption and associated adverse effects, and shortens recovery time and hospital stay.
Senese and Kovacs evaluated the risks posed by bilateral IANB and found that bilateral IANB is a safe technique that enhances our patient's quality of life, and the problems linked to it are typically exaggerated and unfounded. The results of this study correlate with our results regarding the safety and efficacy of the block with rare complications from bilateral IANB (16).
Being one of the rare studies on peripheral nerve blocks in fixation of fracture mandibular under general anesthesia intraoperatively and postoperatively, our study has this advantage. The IANB has a failure rate of approximately 15%, which could be diminished with accurate needle placement, since a frequent error involves inserting the needle excessively anteriorly or posteriorly to the target location, along with the utilization of ultrasound-guided IANBs (17, 18). A small sample size was taken, and this could be regarded as our first stepping stone in this direction, and few studies were using IANB under general anesthesia in fracture mandibular surgeries. More investigations evaluating the intensity and duration of the analgesic effect of bilateral IANB are needed to augment our findings.
5.1. Conclusions
Bilateral IANB can be a safe and efficient method to reduce the amount of opioid medications required and manage postoperative pain and discomfort in mandibular fracture surgeries.