1. Background
Chronic pain involves repeated activation of N-methyl D-Aspartate receptors (NMDAR) (1). In in vitro studies, NMDAR activation occurs in response to its binding with glycine or NMDA (2). The NMDAR activation triggers cytosolic calcium influx, a secondary messenger in the chronic pain pathway through the mitogen-activated protein kinase (MAPK) (3, 4). Within the MAPK pathway, phosphorylation of extracellular signal-regulated kinase (ERK) serves as a key enzyme for neuron activation and central sensitization (5, 6). Elevated levels of phosphorylated ERK contribute to enhanced neuronal gene transcription and long-term synaptic plasticity (7).
The dorsal root ganglion (DRG) is a target for chronic pain management, as it contains neurons involved in the pain pathway (8). During pain, neurons undergo rapid activation, accompanied by an increased energy demand to support enhanced energy production (9). Chronic pain also causes disrupted calcium homeostasis, impairing mitochondrial functions and linking mitochondria to pain mechanisms as key calcium regulators (10). Activated neurons must maintain resting membrane potential, relying on ATP to power ion pumps like the sodium-potassium pump, which regulate sodium and potassium gradients essential for generating action potentials (11).
Pulsed radiofrequency (PRF), using a frequency range of 500 kHz, is an established method for managing pain by targeting neurons in the DRG with minimal tissue damage and fewer side effects (12-14). Clinically, it has been observed to relieve various types of pain, including neck pain, occipital neuralgia (15, 16), back pain (16), lumbar facet joint pain (17), radicular pain (18), and myofascial pain (19). Despite its clinical use, the mechanisms underlying PRF's effect on chronic pain remain under investigation, with theoretical models suggesting the modulation of multiple pain pathways (20). Some hypotheses suggest that PRF alleviates pain by creating an electric field that affects neuronal membrane potential, cellular function, and organelle activity (21).
Our previous study explored the effects of 2 Hz PRF on dorsal root neuron activity induced by NMDA. The findings showed that 2 Hz PRF reduces neuron sensitization by modulating cytosolic calcium influx, mitochondrial membrane potential (MMP), and cytosolic ATP levels (22). In clinical practice, 2 Hz and 4 Hz are commonly applied PRF frequencies. Our previous study on healthy DRG neurons showed that both frequencies preserved neuronal physiological conditions with no significant differences from unexposed neurons, indicating PRF's safety for neuronal activity (23). However, limited scientific data are available that directly compare the effects of these frequencies on neuronal activity.
2. Objectives
This study aimed to compare the effects of 2 Hz and 4 Hz PRF on cytosolic calcium dynamics, MMPs, and neuronal activity in activated DRG neurons.
3. Methods
3.1. Study Design
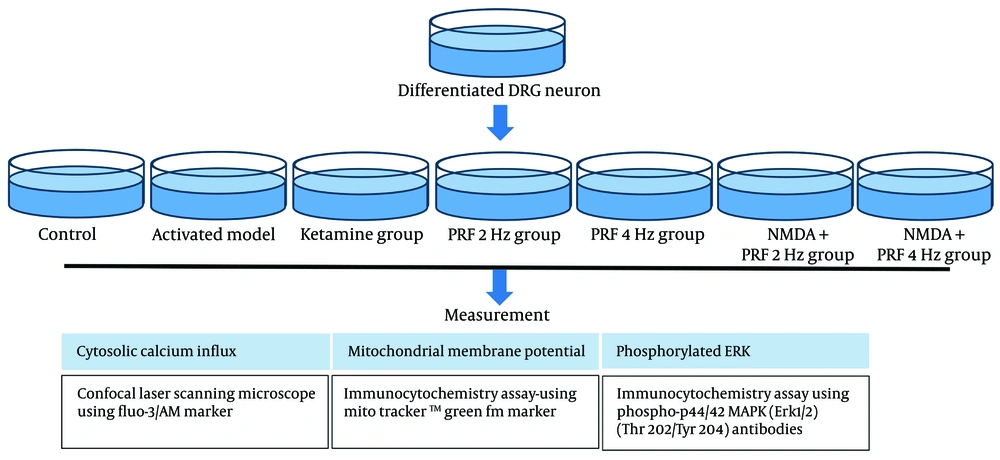
This study employed a true experimental design using DRG neuron cultures derived from the F11 cell line (08062601, Sigma, USA) to compare the effects of PRF at 2 and 4 Hz on activated DRG neurons. The study methods were reviewed and approved by the Faculty of Medicine, Brawijaya University Ethical Clearance Committee (approval No. 137/EC/KEPK/08/2024), ensuring adherence to the highest ethical standards.
3.2. Neuron Culture
The cell culture method, as described by Hashemian et al., involved the differentiation of the F11 cell line into DRG neurons, based on the approach by Pastori et al. (24), with modifications. Cells at a concentration of 2.5 × 105 cells/mL were cultured for 24 hours in six-well plates using Ham F-12 medium. Subsequently, the medium was replaced with a differentiation medium consisting of DMEM, 1% FBS, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. The medium was changed every 2 days, and the cells were cultured for 9 to 14 days until the morphology resembled neurites. Differentiated DRG neurons derived from the F11 cell line were assessed using morphological and biomolecular approaches (23). N-methyl D-Aspartate was used to activate NMDAR specifically in DRG neurons. Consistent with our previous study, DRG neuron activation was achieved by adding NMDA at a concentration of 80 µM to the cell culture.
3.3. Study Groups
The experiment included seven treatment groups, each with three plate replications. The first group served as the control, while the second group received NMDA 80 µM to induce neuron activation. The third group was treated with Ketamine at 100 µM as an NMDAR blocker, to confirm that NMDA induction specifically targets NMDAR. The fourth and fifth groups received PRF at 2 Hz (20 ms for 360 seconds) and 4 Hz (10 ms for 360 seconds), respectively. The sixth and seventh groups were exposed to PRF at 2 Hz (20 ms for 360 seconds) and 4 Hz (10 ms for 360 seconds) following NMDA induction (Figure 1). The PRF frequency and duration were based on clinical relevance (25, 26). Pulsed radiofrequency was applied using an RF generator by Cosman (Cosman Medical Company, USA), following the PRF protocol outlined in a previous study by Laksono et al. (23).
3.4. Cytosolic Calcium Influx, Mitochondrial Membrane Potential, and Neuron Activity Measurement
Neuron activation was assessed by measuring phosphorylated extracellular signal-regulated kinase (pERK) levels using an immunocytochemistry assay with Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) antibodies (36-8800, Thermo Fisher Scientific, USA). Calcium levels were measured using a confocal laser scanning microscope with a Fluo-3/AM marker (F4897-1MG, Sigma-Aldrich, USA), comparing basal and treatment levels as described by Pan et al. (27). The basal calcium level was measured over 1 minute, while treatment levels were assessed over 9 minutes. Mitochondrial membrane potential (MMP) was measured using a MitoTracker™ Green FM marker (M7514, Thermo Fisher Scientific, USA) through an immunocytochemistry assay, following the method of Pendergrass et al. (28).
3.5. Statistical Analysis
The effects of PRF at 2 and 4 Hz on MMP and pERK were assessed using a one-way ANOVA, followed by a Tukey post hoc test. Cytosolic calcium dynamics were displayed in a time-series graph to represent real-time dynamics. Statistical analysis was performed with a 5% significance level (α = 0.05) and 95% confidence intervals, utilizing SPSS version 20.0 (IBM Statistics, USA).
4. Results
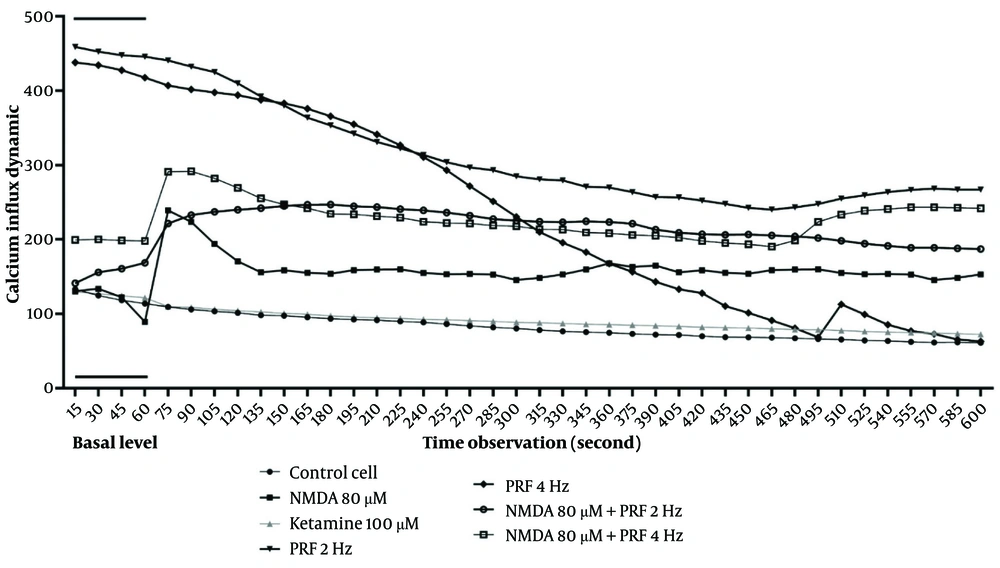
Calcium dynamics were measured over a 10-minute period (600 seconds), with the first minute serving as the baseline level, during which no intervention was applied. Real-time calcium assessments indicated that control neurons maintained stable calcium levels. In contrast, activated DRG neurons induced by NMDA exhibited a substantial increase in cytosolic calcium. Pulsed radiofrequency at both 2 Hz and 4 Hz resulted in a gradual decline in cytosolic calcium over time. However, when PRF was applied to the activated DRG neuron model, calcium levels increased above baseline. The PRF at 2 Hz demonstrated more stable calcium dynamics compared to 4 Hz, with a consistent decrease observed over the measurement period (Figure 2).
Cytosolic calcium dynamic. Activated dorsal root ganglion (DRG) neurons show a significant cytosolic calcium influx. Pulsed radiofrequency (PRF) 2 and 4 Hz on DRG neurons show a decline in cytosolic calcium. Pulsed radiofrequency 2 and 4 Hz exposure on activated DRG neurons show calcium elevation. However, 2 Hz shows a more stable dynamic than 4 Hz.
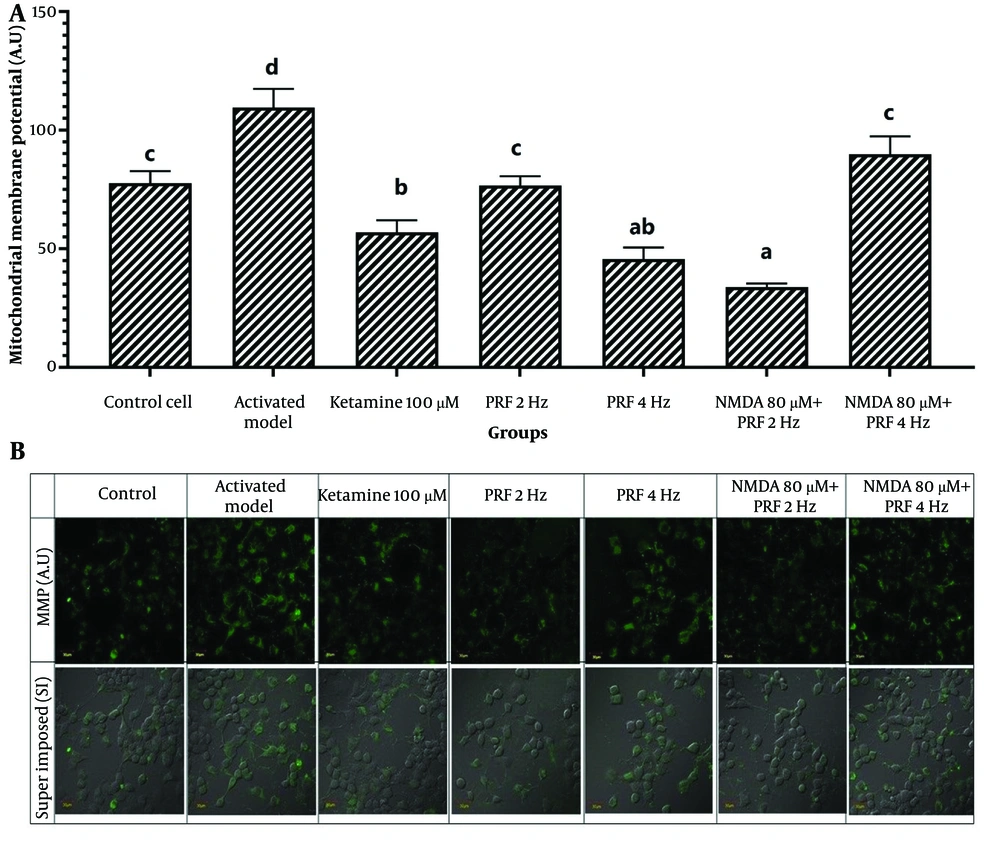
Mitochondrial membrane potential measurements revealed significant differences among the groups (P = 0.000) (Table 1). Post hoc analysis showed that MMP was significantly elevated in the activated DRG neuron model (109.60 ± 7.82; 95% CI: 100.75, 118.45) compared to control cells (77.64 ± 5.13; 95% CI: 71.83, 83.45) (P = 0.000). In DRG neurons exposed to PRF at 2 Hz (76.64 ± 3.93; 95% CI: 72.19, 81.09), there was no significant difference compared to control cells (P = 1.000), whereas exposure at 4 Hz resulted in a significant decrease (45.61 ± 4.93; 95% CI: 40.03, 51.19) (P = 0.000). In the activated neuron model, PRF at 4 Hz (89.80 ± 7.60; 95% CI: 81.20, 98.40) showed no significant difference in MMP relative to control cells (P = 0.169), while PRF at 2 Hz induced a significant reduction (33.87 ± 1.46; 95% CI: 32.22, 35.52) (P = 0.000). Compared to the activated DRG neuron model, both 2 Hz and 4 Hz PRF significantly reduced MMP (P = 0.000 and P = 0.009, respectively) (Figure 3).
| Treatment Group | Mean ± SD (95% CI) | P-Value |
|---|---|---|
| Control cell | 77.64 ± 5.13 (71.83, 83.45) | 0.000 a |
| Activated model | 109.60 ± 7.82 (100.75, 118.45) | |
| Ketamine | 56.88 ± 2.95 (53.54, 60.22) | |
| PRF 2 Hz | 76.64 ± 3.93 (72.19, 81.09) | |
| PRF 4 Hz | 45.61 ± 4.93 (40.03, 51.19) | |
| NMDA + PRF 2 Hz | 33.87 ± 1.46 (32.22, 35.52) | |
| NMDA + PRF 4 Hz | 89.80 ± 7.60 (81.20, 98.40) |
Abbreviations: PRF, pulsed radiofrequency; NMDA, N-methyl D-Aspartate.
a Significant different in one way ANOVA test with P < 0.05.
Potential membrane mitochondria. A, activated dorsal root ganglion (DRG) neurons show significant elevation. Pulsed radiofrequency (PRF) 4 Hz on activated DRG neurons shows a significant decline, while 4 Hz shows no difference with control cells. B, the fluorescent image of the potential membrane mitochondria marker. * Superimpose (SI): A picture of the results of combined observations from MMP expression and DIC (differential interference contrast) observations. Magnification: 400X. The notation abc indicates statistical differences between groups based on Post Hoc test
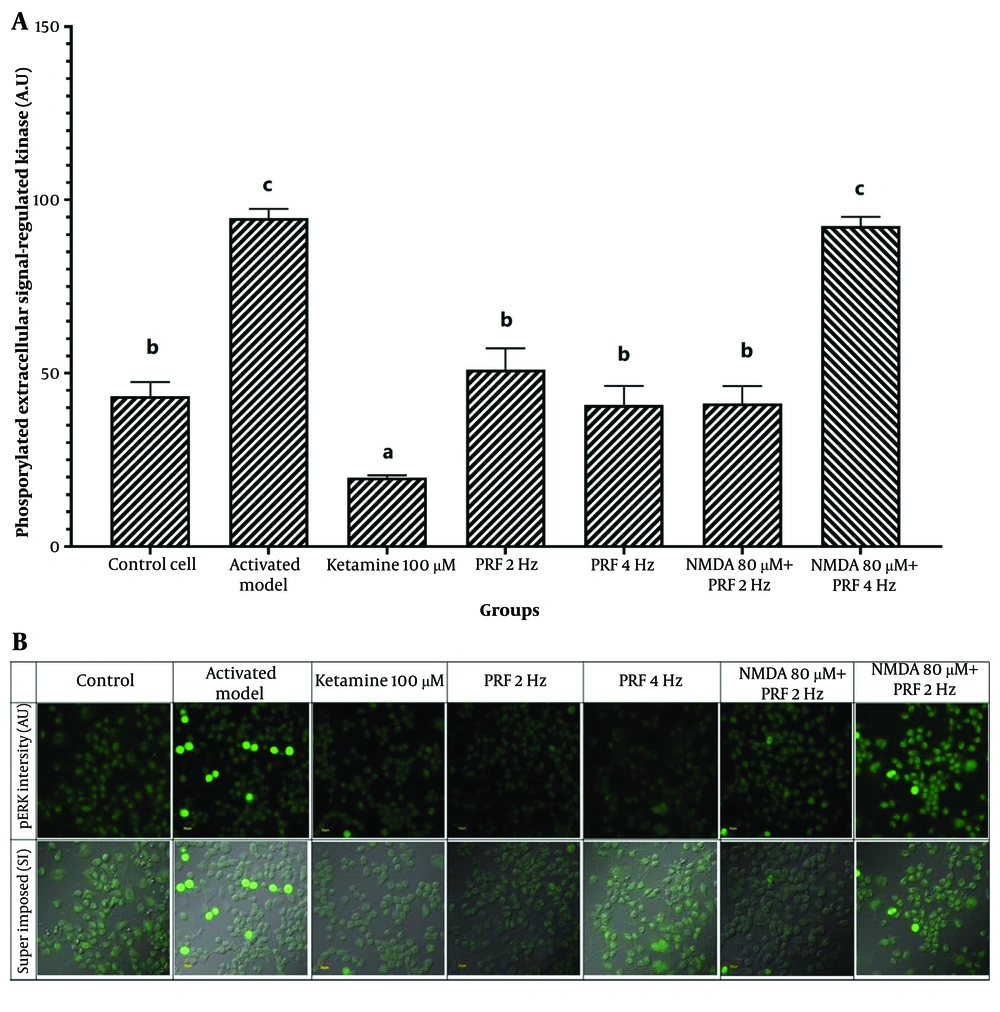
The DRG neuron activation is a key physiological process in chronic pain, contributing to the transmission of pain signals to the central nervous system. This study measured DRG neuron activation as a parameter influenced by PRF in chronic pain management interventions. Measurement of pERK levels showed a significant difference among groups (P = 0.000) (Table 2). Post hoc analysis indicated that activated neurons induced by NMDA exhibited a significant increase in pERK (94.80 ± 2.62; 95% CI: 91.84, 97.76) compared to control cells (43.37 ± 4.07; 95% CI: 38.76, 47.98) (P = 0.000). Ketamine exposure resulted in the lowest pERK intensity. Exposure to PRF at both frequencies in DRG neurons showed no significant difference from the control cells (P > 0.05). However, PRF at 4 Hz exposure (92.47 ± 7.60; 95% CI: 83.87, 101.07) in activated neurons did not significantly differ from the activated model (P = 0.992). The PRF at 2 Hz exposure to the activated neuron produced a significant reduction in pERK levels (41.27 ± 5.06; 95% CI: 35.54, 47.00) compared to the activated model, indicating a significant decrease in neuron activity (P = 0.000) (Figure 4).
| Treatment Group | Mean ± SD (95% CI) | P-Value |
|---|---|---|
| Control cell | 43.37 ± 4.07 (38.76, 47.98) | 0.000 a |
| Activated model | 94.80 ± 2.62 (91.84, 97.76) | |
| Ketamine | 19.89 ± 0.65 (19.15, 20.63) | |
| PRF 2 Hz | 51.05 ± 6.15 (44.09, 58.01) | |
| PRF 4 Hz | 40.77 ± 5.60 (34.43, 47.11) | |
| NMDA + PRF 2 Hz | 41.27 ± 5.06 (35.54, 47.00) | |
| NMDA + PRF 4 Hz | 92.47 ± 7.60 (83.87, 101.07) |
Abbreviations: PRF, pulsed radiofrequency; NMDA, N-methyl D-Aspartate.
a Significant difference in one way ANOVA test with P < 0.05.
Dorsal root ganglion (DRG) Neuron activity. A, pulsed radiofrequency (PRF) 2 and 4 Hz exposure to DRG neurons show no significant elevation of neuron activity. Pulsed radiofrequency 2 Hz exposure to the activated DRG neuron shows no significant difference with the control cell, while 4 Hz shows a significant elevation of neuron activity; B, the fluorescent image of phosphorylated extracellular signal-regulated kinase (pERK). * Superimpose (SI): A picture of the results of combined observations from pERK expression and DIC (differential interference contrast) observations. Magnification: 400X. The notation abc indicates statistical differences between groups based on Post Hoc test
5. Discussion
The mechanism of PRF raises several questions, particularly regarding whether different frequencies, such as 4 Hz and 2 Hz, have the same effect, given that electromagnetic waves affect neuronal communication (29). Activated neurons exhibit cytosolic calcium elevation, driven by the activation of NMDA receptors, which facilitate substantial calcium influx (30). Exposure to PRF in activated DRG neurons initially increases cytosolic calcium levels compared to baseline. However, 4 Hz PRF induces a gradual decline over time, whereas 2 Hz PRF maintains relatively stable calcium levels after the initial peak. Cytosolic calcium influx is associated with activated/sensitized neurons (31).
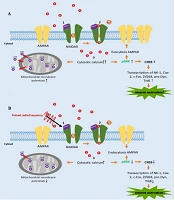
Calcium activates ERK through an upstream cascade involving PKC, Raf-1, and MEK (4). It also stimulates CaMKII, enhancing AMPAR conductivity and membrane transport, contributing to chronic pain (32). The PRF exposure in activated neurons raises cytosolic calcium levels, though less than in the activated model. Calcium balances kinases and phosphatases in neuronal physiology and is related to long-term potentiation (LTP) and long-term depression (LTD) regulation. High calcium levels activate kinases like CaMKII, phosphorylating AMPA receptors to enhance synaptic strength and promote LTP, while low calcium levels activate phosphatases like calcineurin, reducing receptor efficacy and inducing LTD (33). Figure 4 illustrates the schematic difference in calcium levels. This mechanism may underlie the PRF effect on calcium dynamics in activated DRG neurons.
The increase in cytosolic calcium in activated neurons is linked to mitochondrial activity (34). We examined how cytosolic calcium levels influence MMP and observed a significant increase in the membrane potential of activated DRG neuron models. Literature suggests that specific intracellular calcium levels can differentially impact mitochondrial function, particularly energy production (35). Szibor et al. (36) found that elevated intracellular calcium increases MMP through malate-aspartate shuttle (MAS) activation without significantly impacting mitochondrial calcium uptake. In the activated neuron, there is a high energy demand, which responds by increasing energy production initiated by calcium influx (9).
We also found that PRF at 2 and 4 Hz could significantly reduce the MMP in activated neurons. This reduction in MMP could reflect a decrease in neuronal activity, manifesting as a decrease in energy demand. No study has compared the effect of PRF at 2 and 4 Hz on MMP dynamics. Therefore, this study paves the way for further research that could more specifically assess the direct mechanism of PRF on MMP.
To understand neuron activity, we analyzed pERK intensity, as this enzyme can indicate neuron activation or sensitization (5). This study found a significant increase in pERK in activated DRG neuron models. Phosphorylated ERK translocates to the nucleus to activate several transcription factors, including cAMP-response element-binding protein (CREB) (37), which plays a role in the transcription of genes that trigger long-term neural plasticity (37).
Interestingly, PRF at 4 Hz did not reduce the activity of activated DRG neurons, while 2 Hz decreased pERK back to normal levels. This experimental result provides insight into pain management practices. Our study found that although PRF at 4 Hz could reduce the MMP, this frequency fails to reduce neuron activity concerning pERK intensity. This study provides molecular evidence of one of the PRF mechanisms in ameliorating pain. However, a higher frequency, 4 Hz, is unable to reduce pERK levels in sensitized/activated DRG neurons.
This study raises a discussion, as PRF at 4 Hz could clinically reduce several pain cases, including successfully reducing pain in 80% of patients with trigeminal neuralgia (26). The difference in pain targets may contribute to the different results. This study uses DRG neurons, where most chronic neuropathic pain arises (38). Moreover, several factors may contribute to this event. In this study, PRF at 4 Hz may fail to reduce calcium influx since pERK expression depends on the neuron's calcium concentration. A previous study hypothesized that PRF could modulate electroporation, affecting the calcium channel and modulating calcium influx (39). Previous studies also state that PRF could affect ion channels in the DRG neuron, such as Na/K channels, and affect action potential (40).
However, further studies exploring the effect of different frequencies of PRF have not yet been conducted on activated neuron models. Therefore, further research could be established to assess the specific mechanism of PRF in electroporation or ion channel modulation.
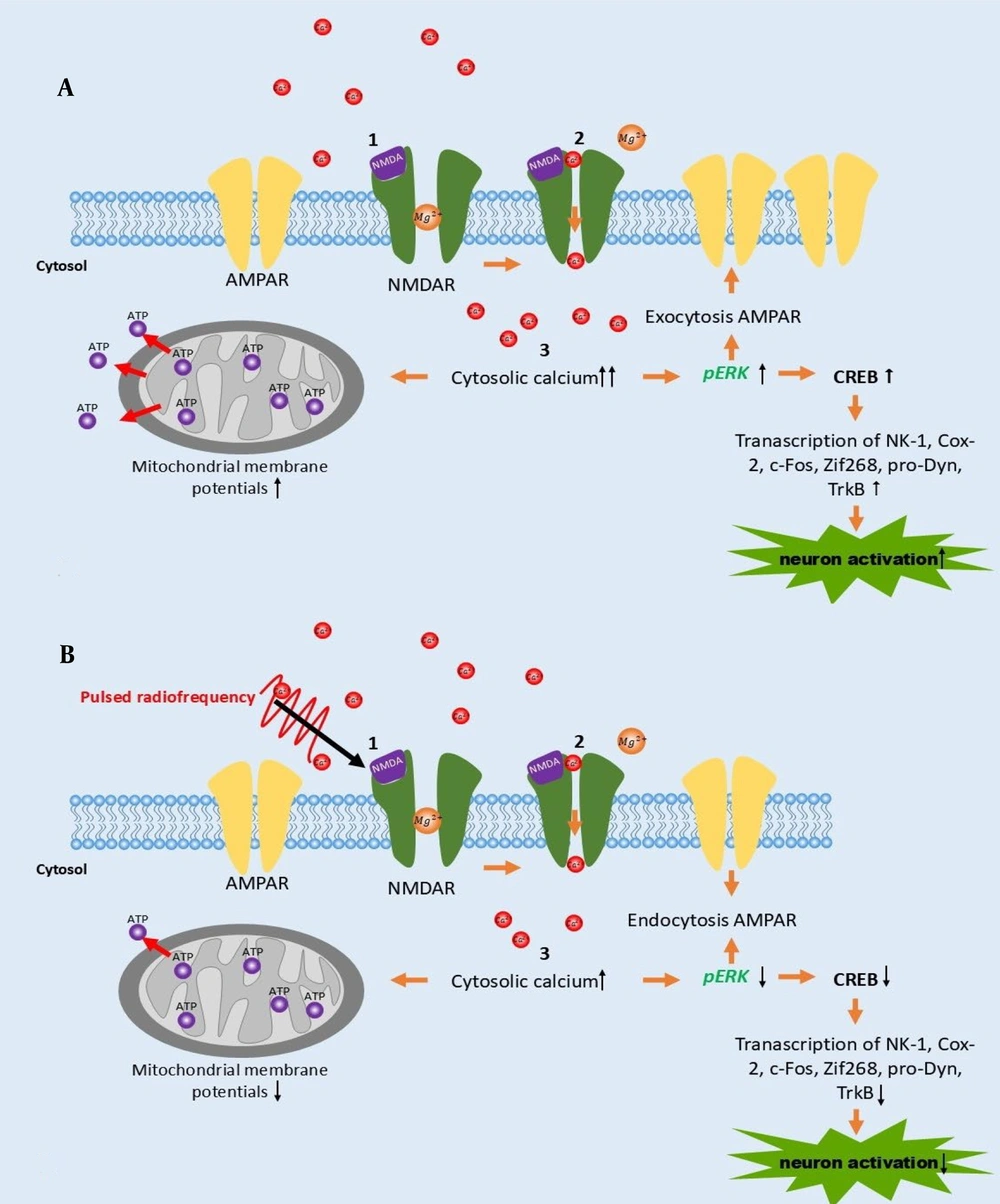
This is the first in vitro study to compare the effects of PRF at 2 and 4 Hz in sensitized DRG neurons, providing insight into the molecular analysis of the PRF mechanism in pain management. Based on our study, we summarize the mechanism of PRF in Figure 5. PRF exposure facilitates an increase in calcium influx, although less than in activated DRG neurons induced by NMDA. This mechanism is followed by a reduction in MMP and pERK intensity (Figure 5B). According to references, the reduction of pERK results in decreased CREB, which is implicated in reduced neuron activation (5). This mechanism could enrich the latest knowledge of the PRF mechanism of action.
A, the schematic mechanism of neuron activation following NMDA induction; B, pulsed radiofrequency (PRF) exposure on activated neurons promotes calcium influx, which works backward by decreasing phosphorylated extracellular signal-regulated kinase (pERK) expression, which manifests in a decrease of neuron activation and mitochondrial membrane potentials (MMP).
In studying the molecular mechanisms underlying PRF therapy at different frequencies, it becomes evident that PRF at 2 Hz offers distinct advantages over 4 Hz. The study elucidates that while both frequencies affect various neuronal activities induced by NMDA, PRF at 2 Hz demonstrates a more favorable modulation of critical biomarkers associated with neuronal sensitization. Specifically, the observed reduction in pERK levels following exposure to PRF at 2 Hz indicates mitigated neuronal sensitization, a crucial aspect in chronic pain management. This study also provides evidence that PRF at 2 Hz is more suitable for intervening in pain related to DRG neurons compared to 4 Hz. This finding supports a review by Negro et al. (41), which found that PRF at 2 Hz applied to the DRG of patients with radicular pain is safe and effective in reducing pain. Further studies can be conducted to analyze PRF in other types of pain.
However, this study has several limitations, including measuring pERK and MMP in cross-section. Measurement at serial time points, such as 1-minute intervals, would be beneficial to show the dynamics of these parameters and provide more detailed insights into the effect of PRF in ameliorating pain. Moreover, measuring mitochondrial calcium influx is also required to show the direct correlation to cytosolic calcium influx following neuron activation. Mitochondrial calcium influx can be measured using Rhod-2/AM dye and analyzed using two-dimensional laser-scanning microscopy (42).
5.1. Conclusions
Pulsed radiofrequency at different frequencies affects cytosolic calcium, mitochondrial physiology, and neuronal activity dynamics in activated DRG neurons in response to radiofrequency. Both frequencies increase cytosolic calcium in the neurons, but to a lesser extent than in activated neurons, and reduce MMP. Pulsed radiofrequency at 2 Hz is able to reduce neuronal activity back to normal levels, while 4 Hz fails to achieve this effect.