1. Context
The decline in cognitive function, including memory, attention, and executive function, after surgery, especially in elderly patients, is termed post-operative cognitive dysfunction (POCD) (1). While the precise pathophysiology of POCD remains complex and not fully understood, emerging evidence suggests that various factors, including inflammation, anesthesia, and comorbidities, may contribute to its development (2). However, one often overlooked aspect in the study of POCD is the role of sensory impairments, particularly olfactory dysfunction, in exacerbating or triggering cognitive decline following surgery (3).
Olfactory impairment, or dysfunction in the sense of smell, has long been recognized as a marker of neurological conditions such as Alzheimer’s disease, Parkinson’s disease (PD), and other forms of dementia (4, 5). Olfaction plays an essential role in memory formation and emotional regulation, as the olfactory system is directly linked to the limbic system, a brain region involved in memory processing and emotional responses (6). Disruptions in olfactory function can lead to a cascade of neurological consequences, including altered mood, anxiety, and cognitive decline, potentially setting the stage for more severe forms of neurodegeneration (7).
The potential mechanisms linking olfactory dysfunction to cognitive decline primarily involve shared pathologies affecting both olfactory and cognitive function, leading to the disruption of key neural pathways such as the entorhinal-hippocampal circuit (8). This connection between the olfactory system and cognitive function makes it plausible that olfactory dysfunction could be a contributing factor or a predisposing marker for POCD (9). Despite growing interest in understanding how sensory impairments, including olfactory dysfunction, impact cognitive health, relatively few studies have systematically explored the relationship between olfactory impairment and POCD (3).
Olfactory dysfunction is a common but often underreported condition in the perioperative setting, yet its potential to influence postoperative outcomes could be significant. The influence of olfactory impairment on cognitive function following surgery may not only complicate recovery but also increase vulnerability to long-term cognitive decline, particularly in older adults who are already at heightened risk for cognitive impairment (10). The importance of studying the interaction between olfactory impairment and POCD lies in the potential for early detection, intervention, and improved management strategies for patients undergoing surgery. For example, identifying olfactory dysfunction preoperatively could help clinicians anticipate the likelihood of postoperative cognitive issues and take preventive measures (11). Additionally, understanding how olfactory impairment influences cognitive outcomes could inform the development of targeted therapeutic strategies to minimize cognitive decline during recovery (12).
Given the significant burden that POCD places on both patients and healthcare systems, addressing this gap in the literature is essential. A systematic review of existing studies examining the impact of olfactory dysfunction on postoperative cognitive outcomes can provide valuable insights into this underexplored area of research. It may also reveal whether olfactory testing could serve as a predictive tool for identifying patients at higher risk for developing POCD and whether interventions aimed at restoring or compensating for olfactory function might improve cognitive recovery post-surgery.
2. Objectives
The present study aimed to assess the association between olfactory impairment and POCD, exploring whether olfactory dysfunction serves as a risk factor, a diagnostic marker, or an indicator of postoperative cognitive trajectory. By critically evaluating the available literature, this review seeks to clarify how olfactory impairment may contribute to POCD and POD and highlight areas for further investigation.
3. Methods
3.1. Search Strategy
A systematic review adhering to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines was conducted (13). From inception until December 2024, PubMed, Scopus, and WOS were thoroughly searched. The search combined terms related to "olfactory dysfunction", "postoperative cognitive impairment" and "delirium". No language restrictions were applied. Two independent reviewers manually screened the reference lists of included studies to identify additional eligible studies not retrieved through the database search (Table 1).
| Database | Search Strategy |
|---|---|
| PubMed | ["olfactory dysfunction"(MeSH) OR "olfactory impairment" OR "anosmia" OR "hyposmia"] AND ["postoperative cognitive dysfunction"(MeSH) OR "postoperative cognitive impairment" OR "postoperative delirium" OR "cognitive decline"] |
| Scopus | [TITLE-ABS-KEY ("olfactory dysfunction" OR "olfactory impairment" OR "anosmia" OR "hyposmia")] AND [TITLE-ABS-KEY ("postoperative cognitive dysfunction" OR "postoperative delirium" OR "cognitive impairment")] |
| WOS | TS = ("olfactory dysfunction" OR "olfactory impairment" OR "anosmia" OR "hyposmia") AND TS = ("postoperative cognitive dysfunction" OR "postoperative delirium" OR "cognitive impairment") |
Abbreviation: WOS, Web of Science.
3.2. Eligibility Criteria
Studies were included if they: (1) Examined the relationship between olfactory dysfunction and cognitive outcomes, (2) used validated tools to evaluate both olfactory and cognitive function, and (3) involved patients undergoing major operations requiring significant anesthesia time (e.g., cardiac, abdominal, neurosurgery). Studies were excluded if they: (1) Lacked quantitative data, (2) were editorials, commentaries, conference abstracts, or case reports, (3) were published in languages other than English, (4) focused on non-surgical populations, (5) were animal studies or in vitro experiments, and (6) lacked postoperative cognitive assessments within the specified period.
3.3. Study Selection
The selection process comprised three phases: Title and abstract screening, full-text appraisal, and final inclusion. Two independent reviewers reviewed all records based on titles and abstracts. Studies passing the first screening underwent full-text evaluation, and those not meeting the eligibility criteria were excluded. The two reviewers resolved differences through discussion or consultation with a third unbiased reviewer.
3.4. Data Extraction and Quality Assessment
After screening, data extraction was conducted by two authors, including details such as author, year, study design, population, sample size, duration of follow-up, olfactory and cognitive tests used, and main findings. Disagreements were resolved through third-party intervention. The modified Newcastle-Ottawa Scale (NOS) for observational studies was used to assess study quality (14). This instrument evaluates methodological quality by assessing studies in three domains: Selection of participants, comparability of study groups, and evaluation of outcomes/exposure. Each study received a quality score between 0 and 9, with higher ratings indicating reduced bias risk. Two reviewers independently assessed the risk of bias; differences were settled through discussion or third-reviewer consultation. Our data synthesis included the results of the risk of bias assessment to interpret study results more precisely. Studies with a higher risk of bias were discussed with caution, emphasizing potential limitations that could compromise the validity of their results (Table 2).
3.5. Data Synthesis
Due to the heterogeneity in study designs, olfactory and cognitive tests, follow-up durations, and study effect sizes, a narrative synthesis was performed. Study characteristics were summarized using descriptive statistics, and key findings were qualitatively synthesized.
4. Results
4.1. Study Selection
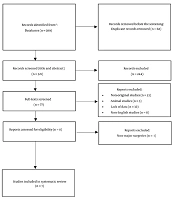
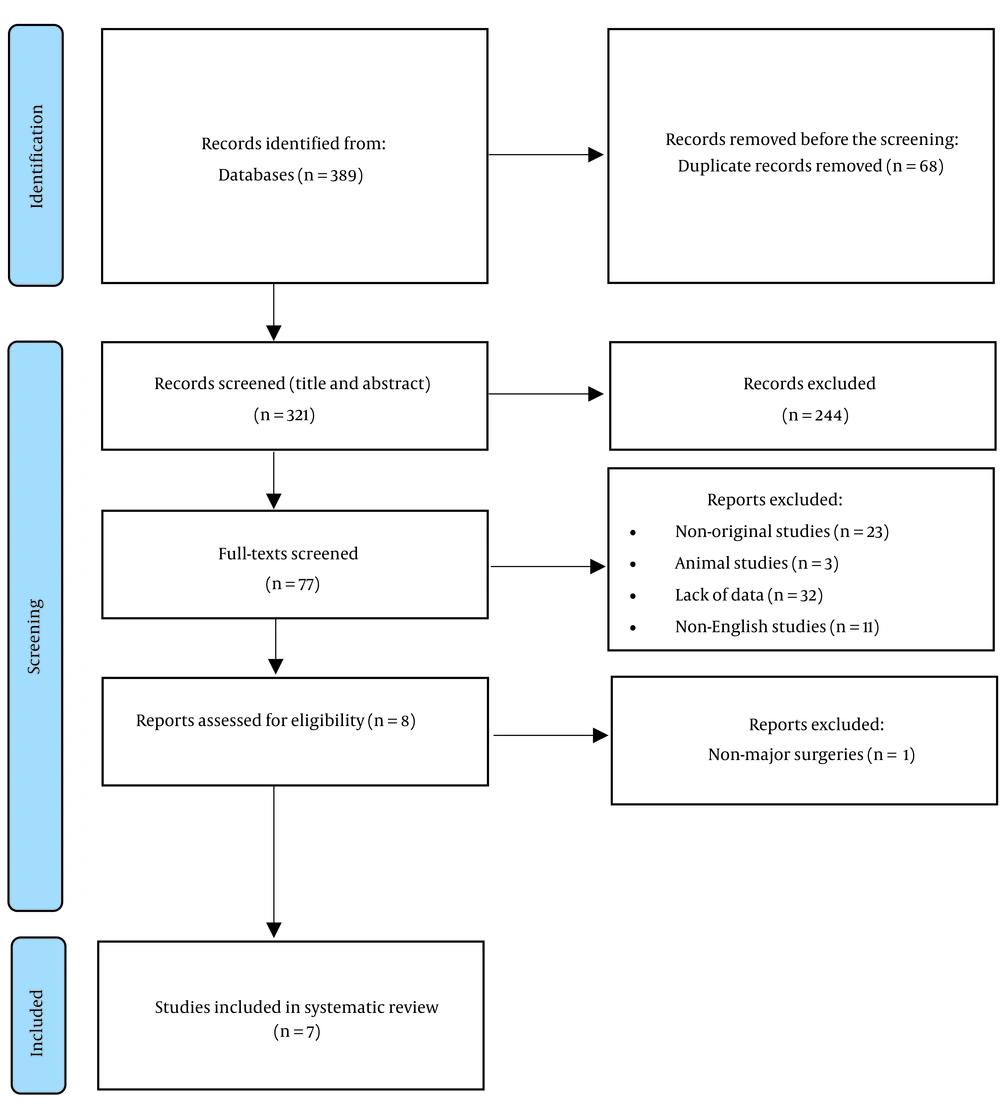
Our electronic database search, including PubMed, Scopus, and WOS, resulted in 389 articles. After eliminating duplicate entries, 321 articles were evaluated for relevance based on their titles and abstracts. Of these, 77 were chosen for a comprehensive full-text review. Following a thorough assessment, seven studies fulfilled all inclusion criteria and were consequently selected for systematic review (Figure 1).
4.2. Study Characteristics
Seven studies, including 1,038 participants, were conducted between 2004 and 2022 in diverse settings in the UK, USA, South Korea, China, and Belgium, with sample sizes ranging from 34 to 229 participants. Participants in the included studies underwent major surgeries, including cardiac, abdominal, and neurosurgery. The mean age was 69.65 ± 7.56 years. Regarding gender distribution, there was a slight predominance of males, reflected in the male-to-female ratios in the included studies. Follow-up periods varied from immediate postoperative evaluations (e.g., 3 days) to one year.
In the included studies, olfactory function was predominantly assessed using the sniffin’ sticks test (a 12-item identification test), the brief smell identification test (BSIT), or the cross-cultural smell identification test (CCSIT). Cognitive outcomes were assessed using various measures, with the most prevalent being the mini-mental state examination (MMSE), confusion assessment method (CAM), and clock drawing test (CDT), among others. The studies employed various designs, including case-control studies (3, 15), prospective cohort studies (16, 18, 19), and cross-sectional studies (17, 20), demonstrating a multifaceted approach to exploring the association between olfactory dysfunction and postoperative cognitive outcomes across diverse populations and surgical settings (Table 3).
| First Author/Country (Ref.) | Study Design | Population | N | Age | Gender (M/F) | Follow-up Duration | Olfaction Test | Delirium Test/Incidence | Cognitive Test | Main Finding | Adjustment/Matching | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rentowl and Hanning/UK (15) | Case-control pilot study | > 60 years old patients scheduled to have major abdominal, orthopedic, or non-cardiac thoracic surgery under general anesthesia with an expected hospital stay of at least 4 days | 229 | 69.46 ± 6 | 126/103 | 7-day and 3-month | Sniffin’ sticks 12-item identification test | - | Visual verbal learning test; concept shifting test; stroop color word interference test; letter-digit coding test | No direct correlation between olfactory dysfunction and POCD | Age, smoking, alcohol intake, gender or MMSE score, and subsequent cognitive | 4 |
| Brown et al./USA (16) | Nested prospective cohort study | Elderly patients who underwent coronary artery bypass and/or valve surgery | 165 | 69.98 ± 8 | 107/58 | 4 - 6 weeks after surgery | BSIT | Validated chart review/31% | Rey auditory verbal learning test; rey complex figure test; controlled oral word association test; symbol digit modalities test; trail making B grooved pegboard test | Independent correlation of diminished olfactory function with the occurrence of POD | Age, history of stroke, randomization status, and baseline cognitive score | 7 |
| Kim et al./South Korea (17) | Cross-sectional study | PD patients who had elective surgery under general anesthesia | 34 | 69.4 ± 9.35 | 15/19 | Not mentioned | CCSIT | CAM/50% | K-MMSE; CDR | Emergence of olfactory dysfunction as a significant predictor of postoperative delirium POD, with diminished olfactory scores strongly correlating with POD relative to controls | Sex, age, PD duration, operation time, and ICU admission | 6 |
| Lan et al./China (3) | Case-control study | Elderly patients who underwent abdominal surgery | 60 | 65.5 ± 13 | 28/32 | 3rd and 7th day post-anesthesia | CCCRC olfactory assessment, including: ODT and OIT tests | - | MMSE; HVLT-R; TMT; SCWT; DSCT; VFT | Higher occurrence of olfactory dysfunction among patients undergoing surgery than control, with no significant changes in cognitive performance, between those with and without olfactory impairment | - | 5 |
| Van Regemorter et al./Belgium (18) | Prospective observational study | Patients > 65 years old and scheduled for inpatient minor, intermediate, or major elective non-cardiac surgery under general anesthesia | 155 | 73.00 ± 7.48 | 61/94 | 1 year | Sniffin’ sticks 12-item identification test | - | CDT | Strong correlation between pre-operative olfactory impairment and inferior cognitive performance | Preoperative olfactory identification function, age, gender and baseline characteristics whose level of significance reached P-value < 0.1 | 9 |
| Zhang et al./China (19) | Prospective observational study | Patients aged between 60 to 85 years who underwent non-cardiac and non-neurological surgery under general anesthesia with expected hospital stay ≥ 5 days | 206 | 68.02 ± 7.08 | 140/66 | 5 to 10 days (average of 7 days) after the anesthesia/surgery | Sniffin’ sticks 12-item identification test, including: OT and OI tests | - | Short story module of the randy memory test; verbal fluency test; trail making test; parts a digit-symbol substitution test; digit span subtest of WAIS-Revised; finger tapping test; grooved pegboard test; (dominant and nondominant) block test | Significant association between olfactory dysfunction and dNCR | Age, sex, education, preoperative MMSE score, and days of postoperative neuropsychological tests | 7 |
| Kamath et al./USA (20) | Cross-sectional study | Patients aged > 55 years who underwent primary or re‐operative coronary artery bypass graft with or without valvular surgery or ascending aorta surgery that required cardiopulmonary bypass | 189 | 70.1 ± 7.6 | 141/48 | 4 days post-operative | BSIT | CAM/44% | Rey auditory verbal learning test; rey complex figure test; controlled oral word association test; symbol digits modalities test; trail making test; grooved pegboard | - | Age, duration of cardiopulmonary bypass, logEuroSCORE, and baseline cognition | 9 |
Abbreviations: PD, Parkinson’s disease; BSIT, brief smell identification test; CCSIT, cross-cultural smell identification test; CCCRC, Connecticut Chemosensory Clinical Research Center; ODT, olfactory detection threshold; OIT, olfactory identification threshold; OT, odor threshold; OI, odor identification; CAM, confusion assessment method; K-MMSE, Korean version of the mini-mental state examination; CDR, Clinical Dementia Rating Scale; MMSE, mini-mental state examination; HVLT-R, Hopkins verbal learning test-revised; TMT, trail making test; SCWT, stroop color word test; DSCT, digit-symbol coding test; VFT, verbal fluency test; CDT, clock drawing test; WAIS, Wechsler Adult Intelligence Scale; POCD, post-operative cognitive dysfunction; POD, postoperative delirium; dNCR, delayed neurocognitive recovery; ICU, intensive care unit.
4.3. Comparison of Main Findings Across Studies
The initial documented attempt to explore a potential link between olfactory impairment and postoperative cognitive impairment was established in a pilot case-control study by Rentowl and Hanning (15). They conducted their study among elderly non-cardiac surgery patients, which revealed no direct correlation between olfactory dysfunction (sniffin’ sticks) and POCD. The findings were reported one week and three months post-surgery. Following one week, Fisher’s exact test indicated no significant correlation between olfactory identification impairment and POCD (P = 0.257). Similarly, three months post-surgery, no correlation was found between olfactory recognition capability and POCD (P = 0.144). Fisher’s exact test also revealed no correlation between olfactory identification capability and POCD across cognitive subtests at one week (P = 1.0) and three months post-operation (P = 0.465).
Later, more sophisticated study designs were used to explore this potential link more intricately. For instance, a nested prospective cohort study by Brown et al. (16) indicated that diminished olfactory function was independently correlated with the occurrence of POD. In their unadjusted analyses, patients exhibiting poor baseline olfaction demonstrated a diminished composite cognitive Z-score at 4 - 6 weeks post-surgery (P = 0.006) compared to individuals with normal olfaction. However, after controlling for age, stroke history, and randomization status, no correlation was seen between poor olfaction and the alteration in cognitive Z-score (P = 0.80). Upon incorporating baseline cognitive status into the adjustment model, the baseline cognitive score influenced the relationship between reduced olfaction and the alteration in the cognitive score post-surgery (P = 0.014).
Subsequent analysis revealed that patients with diminished olfactory function exhibited a more significant fall in composite cognitive Z-score post-operatively when their baseline cognitive scores were low, in contrast to those with intact olfaction. Conversely, at elevated baseline cognitive scores, the reduction in cognitive Z-score was indistinguishable between the undamaged and reduced olfaction cohorts. Impaired olfaction correlated with a 1.90-fold heightened risk of POD after controlling for age, stroke history, randomization, and baseline cognitive Z-score (P = 0.009). Interestingly, this heightened risk of delirium associated with decreased olfaction in this paradigm is analogous to an 18-year increase in age. Moreover, for each 1-unit reduction in the baseline olfaction score, the adjusted risk of delirium escalated by approximately 11% (P = 0.03).
Furthermore, in a study by Kim et al. (17) involving PD patients undergoing elective surgery, olfactory dysfunction (evaluated using the CCSIT) emerged as a significant predictor of POD, with a 50% incidence. Diminished olfactory scores were strongly correlated with delirium relative to controls. Their multivariate logistic regression analysis revealed that olfaction (P = 0.03) and operation time (P = 0.04) are significant predictors of POD development. The receiver operating characteristic (ROC) curve for assessing the diagnostic efficacy of CCSIT in predicting delirium demonstrated an area under the curve (AUC) of 0.822 (P = 0.001).
Lan et al. (3), in their case-control study involving elderly patients undergoing abdominal surgery, found a higher occurrence of olfactory dysfunction among surgical patients than controls. However, they observed no significant changes in cognitive performance, as measured by the MMSE and the Hopkins verbal learning test-revised (HVLT-R), between those with and without olfactory impairment. No significant difference in olfactory function was seen between the operative and control groups before surgery. In contrast, the odor identification test (OIT) scores in the surgical group significantly declined on the 3rd and 7th days post-anesthesia (P < 0.05). The odor detection test (ODT) revealed no statistically significant difference between the two groups (P > 0.05).
A prospective observational study conducted by Van Regemorter et al. (18) in Belgium demonstrated a strong correlation between pre-operative olfactory impairment (evaluated with sniffin’ sticks) and inferior cognitive performance (measured by the CDT) at the one-year follow-up. The results indicated that olfactory dysfunction correlated with performance on the CDT (χ2 = 8.507, P = 0.014). In a multivariate logistic regression model, correcting for education level, anosmia remained a significant predictor of reduced performance on the CDT (P = 0.024).
In another prospective observational cohort, Zhang et al. (19) identified a significant association between olfactory dysfunction and delayed neurocognitive recovery (dNCR) within 1 - 4 weeks postoperatively. Participants with dNCR had lower baseline OI (5.08 ± 4.41 vs. 9.67 ± 2.24) or OT (5.75 ± 3.65 vs. 7.00 ± 2.99) than those without dNCR.
In a cross-sectional study, Kamath et al. (20) examined olfaction and cognition preoperatively and postoperatively. Before surgery, patients with impaired olfaction had lower cognitive z-scores than those with normal olfaction (P < 0.0001). When regarded as a continuous variable, poorer baseline olfactory function exhibited a linear correlation with diminished baseline cognitive performance (P < 0.0001). Four days post-surgery, individuals with pre-existing olfactory impairment had a higher prevalence of POD than those with normal olfaction (P = 0.0015). The adjusted odds of delirium were elevated in olfactory-impaired patients compared to those with normal olfaction after controlling for age, duration of cardiopulmonary bypass, and logEuroSCORE (P = 0.001). Statistical significance persisted after incorporating baseline cognition into the model (P = 0.038). Each unit drop in baseline olfactory score correlated with heightened delirium after adjusting for age, cardiopulmonary bypass duration, and logEuroSCORE (P = 0.03), although the model lost statistical significance upon including baseline cognition as a covariate.
4.4. Synthesis of Findings
The findings from seven studies converge to suggest that olfactory dysfunction is a preoperative marker for postoperative cognitive impairment (POCI) and POD in selected contexts, particularly among the frail, elderly, or neurologically vulnerable populations. Prospective cohort studies (16, 18, 19), which feature larger sample sizes and substantial adjustments for confounders, have reported stronger associations between olfactory impairment and postoperative outcomes. The results from case-control studies were more heterogeneous (3, 15), with significant associations observed in specialized populations, such as patients with PD. Additionally, studies identified olfactory dysfunction as being associated with an increased risk of delirium (17, 20), highlighting its potential usefulness as a preoperative screening tool.
5. Discussion
This study reviews the available evidence on the potential link between olfactory dysfunction and POCD, comprehensively evaluating seven studies that explored this relationship across different surgical settings and patient populations. The findings collectively suggest that olfactory dysfunction may be a useful preoperative marker for postoperative cognitive decline, particularly in vulnerable populations. However, the results across studies varied, and several critical gaps warrant further exploration.
The relationship between olfactory dysfunction and POCD has gained attention in recent years due to the increasing recognition of the olfactory system’s role in cognitive and emotional processing, particularly in the aging population. Several studies in this review, particularly prospective cohort studies, found that impaired olfactory function before surgery was associated with a higher risk of postoperative cognitive outcomes, including delirium and dNCR. For example, Brown et al. demonstrated a robust association between baseline olfactory impairment and an increased risk of POD, with a risk ratio (RR) of 1.90 (95% CI: 1.17 - 3.09) for delirium in patients with poor olfactory function (16). Similarly, Zhang et al. identified a significant correlation between baseline olfactory function and delayed cognitive recovery, with a notable increase in the risk of dNCR in patients with impaired olfaction (19). Le et al. found that impaired olfactory function before surgery predicts POD and cognitive decline, suggesting that olfactory testing could be a risk stratification tool (21).
While several studies corroborate the notion that olfactory dysfunction is associated with POCD and delirium, there is considerable variability in the results. For instance, Rentowl and Hanning found no direct correlation between olfactory dysfunction and POCD in a cohort of elderly non-cardiac surgery patients (15). Similarly, Lan et al. observed a decline in olfactory function post-surgery but failed to find a significant association with cognitive outcomes measured by the MMSE and HVLT-R (3). These contrasting findings suggest that various factors, such as the type of surgery, patient demographics, and cognitive assessment tools used, may influence olfactory dysfunction and cognitive outcomes. It is possible that the effects of olfactory impairment on cognition are subtle and may only become apparent in specific patient populations, such as the elderly or those with pre-existing neurological conditions like PD (19).
A common challenge in interpreting the findings of these studies is the potential influence of confounding factors, such as age, baseline cognitive function, comorbidities, and anesthesia protocols. For instance, Brown et al. showed that when baseline cognitive status was included in the analysis, the relationship between olfactory dysfunction and postoperative cognitive decline was significantly influenced by baseline cognitive scores, suggesting that pre-existing cognitive impairment might exacerbate the impact of olfactory dysfunction on post-surgical outcomes (16). This underscores the importance of controlling for confounding variables to isolate the specific effect of olfactory impairment on postoperative cognitive function.
Similarly, Van Regemorter et al. demonstrated that anosmia (complete loss of smell) was a significant predictor of cognitive impairment on the CDT one year after surgery, even after controlling for education level, a common confounder in studies of cognitive function (18). These findings emphasize the need for studies that account for multiple potential confounders and use well-defined cognitive assessment tools to capture the full spectrum of cognitive changes after surgery.
One of the most compelling findings across the studies was the association between olfactory dysfunction and an increased risk of POD, a common form of acute cognitive dysfunction after surgery. Kim et al. found that olfactory dysfunction was a significant predictor of delirium in PD patients undergoing elective surgery (17). This suggests that olfactory dysfunction may be a marker of broader cognitive vulnerability in populations with pre-existing neurological conditions. Furthermore, studies such as Kamath et al. demonstrated that olfactory impairment could be an early warning sign of post-surgical cognitive disturbances, particularly in the context of frail or elderly patients (20).
The mechanisms underlying the relationship between olfactory dysfunction and POCD remain speculative. Still, they may be rooted in the close connection between the olfactory and limbic systems, which play a crucial role in memory and emotional regulation (22). Disruptions in the olfactory system may reflect broader neurological disturbances, including those in areas responsible for cognitive function. Moreover, olfactory dysfunction could serve as an early indicator of neurodegenerative processes, such as Alzheimer’s disease or PD, which are common in older adults (23, 24). Fatuzzo et al.’s study showed that olfactory deficits are early indicators of neurodegenerative diseases, linking them to cognitive decline (25). Zhang et al. reported that increased levels of interleukin-6 (IL-6) in the blood and nasal epithelium post-surgery are linked to olfactory and cognitive impairments (19). Additionally, Bothwell et al. demonstrated that olfactory dysfunction is associated with reduced volumes in brain regions like the hippocampus and olfactory-related areas, which are vital for cognitive processing (26).
Inflammation, anesthesia, and other surgical stressors are also believed to contribute to both olfactory dysfunction and cognitive decline after surgery. For example, a systematic review conducted by Brown et al. suggested that diminished olfactory function might be a marker of heightened vulnerability to inflammation or other stress responses, which could exacerbate postoperative cognitive decline (16). This reasoning underscores the importance of exploring inflammatory and neurological pathways as potential links between olfactory dysfunction and POCD.
This systematic review suggests that olfactory impairment could serve as a useful preoperative predictor of the development of POCD and POD, particularly in older patients and neurologically vulnerable groups. Therefore, routine preoperative olfactory assessments using simple and reasonably priced tests like the BSIT or sniffin’ sticks could help clinicians identify high-risk patients and implement appropriate preventive measures. However, various obstacles to implementation should be considered. These include the need to validate olfactory screening methods in perioperative populations, the absence of standardized olfactory assessment procedures in surgical environments, and insufficient awareness among healthcare practitioners regarding the prognostic relevance of olfactory dysfunction.
Although this systematic review offers valuable insights, certain limitations must be acknowledged. First, the heterogeneity among studies — variations in olfactory assessment tools, cognitive evaluation methods, and follow-up durations — may have affected the comparability of their results. Furthermore, although we accounted for essential confounders, including age and baseline cognitive performance, other potential confounders, such as anesthesia type, surgical time, and perioperative medications, could influence postoperative cognitive outcomes. The lack of consistent reporting of these elements across studies limits our ability to systematically evaluate their impact.
5.1. Conclusions and Future Directions
The present systematic review provides valuable insights into the role of olfactory dysfunction as a potential predictor of postoperative cognitive decline. While the evidence supports a relationship between olfactory impairment and POCD, particularly in vulnerable populations such as older adults and those with neurological conditions, the variability in study findings underscores the need for more robust and homogeneous studies. Future research should aim to standardize assessment tools for both olfactory function and cognitive outcomes, control for confounding factors, and explore potential underlying mechanisms linking these two conditions. Additionally, more extensive multicenter studies and randomized controlled trials are needed to better establish the causal relationship between olfactory dysfunction and postoperative cognitive outcomes.
Exploring the potential for olfactory testing as a preoperative screening tool could have significant clinical implications, offering a simple and cost-effective way to identify patients at higher risk for cognitive complications following surgery. Lastly, therapeutic interventions to improve olfactory function or mitigate its effects on cognition could offer new avenues for enhancing postoperative recovery and quality of life in vulnerable patient populations. Future research could address these gaps and provide more precise guidance on how olfactory dysfunction may be leveraged in clinical practice to reduce the burden of POCD.
References