1. Introduction
Sepsis remains a critical challenge in clinical practice, leading to high morbidity and mortality rates. Current treatments focus on infection control and supportive care but often fail to address the complex pathophysiology of sepsis (1, 2). Emerging research suggests that estrogen, particularly estradiol (E2), could offer novel therapeutic benefits due to its anti-inflammatory, renoprotective, and immune-modulating properties (3).
2. Mechanisms of Estrogen in Sepsis
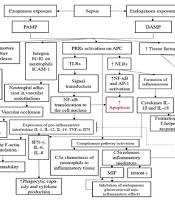
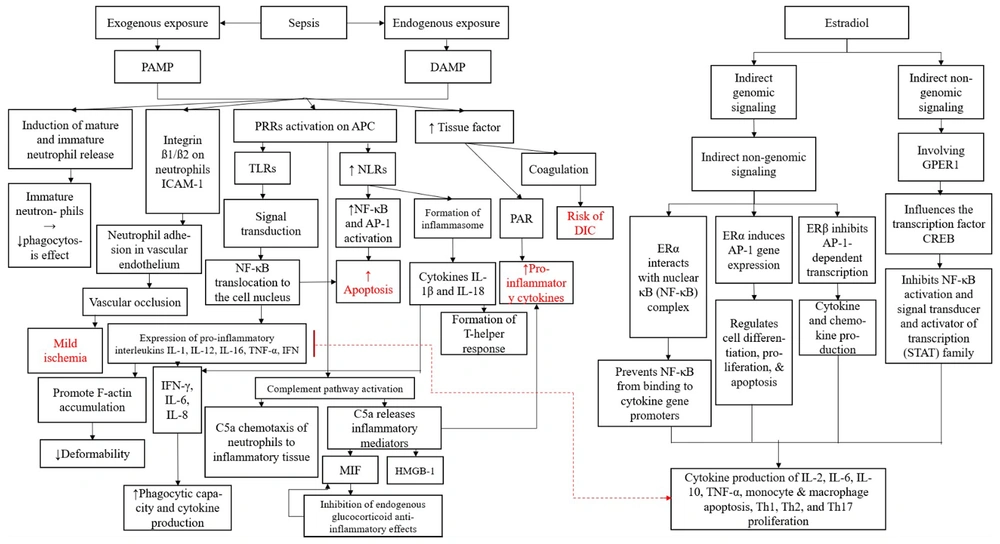
The process begins by categorizing exposure into exogenous [via pathogen-associated molecular patterns (PAMPs)] and endogenous [via damage-associated molecular patterns (DAMPs)], both of which trigger immune responses (4, 5). The PAMPs stimulate neutrophil release, leading to vascular occlusion and ischemia, while DAMPs activate pattern recognition receptors (PRRs), leading to increased nuclear factor-kappa B (NF-κB) activation, inflammasome formation, and pro-inflammatory cytokine production (6-8). This cascade contributes to apoptosis, cytokine release, and potential disseminated intravascular coagulation (DIC) (2, 9). Estradiol is depicted as a modulating factor, exerting both genomic and non-genomic effects to regulate NF-κB, AP-1, and CREB transcription factors, influencing cytokine production, immune cell differentiation, and inflammation resolution (3). Figure 1 highlights key mechanisms underlying immune dysregulation in sepsis and suggests a protective role of E2 against excessive inflammation and coagulation disturbances.
Flowchart illustrating the interplay between sepsis, inflammation, and the regulatory effects of estradiol (E2) on immune and coagulation pathways. Through these mechanisms, E2 modulates the production of key cytokines such as IL-2, IL-6, IL-10, TNF-α, and affects the apoptosis of monocytes and macrophages while influencing Th1, Th2, and Th17 proliferation. This highlights a potential protective role of estrogen in mitigating sepsis-related inflammation and immune dysregulation. Abbreviations: PAMP, pathogen-associated molecular pattern; DAMP, damage-associated molecular pattern; PRR, pattern recognition receptor; TLR, toll-like receptor; NLR, nod-like receptor; NF-κB, nuclear factor kappa B; AP-1, activator protein-1; ICAM-1, intercellular adhesion molecule-1; PAR, protease-activated receptor; DIC, disseminated intravascular coagulation; C5a, a complement system fragment that acts as a potent chemoattractant, recruiting neutrophils to sites of inflammation and enhancing inflammatory mediator release; MIF, macrophage migration inhibitory factor; HMGB-1, high-mobility group box 1; ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; GPER1, G protein-coupled estrogen receptor 1; CREB, cAMP response element-binding protein; STAT, signal transducer and activator of transcription.
3. Sepsis-Anti-inflammatory Effects
Estrogen, particularly E2, has several significant effects in relation to sepsis, including reducing the production of pro-inflammatory cytokines, acting as a renoprotective agent, and enhancing macrophage function (3).
4. Macrophage Function and Trained Immunity
An in vitro study found that E2 can promote trained immunity to facilitate increased macrophage LC3-associated phagocytosis (LAP), which can help eliminate pathogens (10).
5. Renoprotection
Estradiol, especially in combination with 2-methoxyestradiol (2ME), protects against ischemia-reperfusion kidney injury by reducing renal sympathetic nerve activity (11).
6. Molecular Signaling Pathways
Estrogen binds to estrogen receptors ER-α and ER-β, triggering a cascade of intracellular signaling pathways that play key roles in immune modulation and tissue protection (12). These include the mitogen-activated protein kinase (MAPK) pathway, which regulates cell survival and inflammation; nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which controls cytokine production and immune response; peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), which influences mitochondrial biogenesis and metabolic regulation; and heat shock proteins (HSPs), which assist in cellular stress responses. Additionally, estrogen can modulate toll-like receptor 4 (TLR4) signaling, enhancing pathogen recognition, and activate the PI3K/Akt pathway, promoting cellular survival and reducing oxidative stress (13, 14).
7. Gender Dimorphism in Sepsis: A Controversial Perspective
The impact of gender on sepsis outcomes remains debated. Some studies report no difference in mortality (15), while others suggest premenopausal women have better survival due to estrogen’s protective effects (16-19). Rather than questioning if gender affects survival, we should explore why estrogen may improve outcomes. Sun et al. (20) found that E2 enhances macrophage LAP, possibly explaining sex differences in sepsis survival. Their study showed that beta-glucan-induced trained immunity improves sepsis resistance in female mice, but this effect diminishes in ovariectomized (OVX) mice. Beta-glucan increases RUBICON and NOX2 expression in macrophages, boosting reactive oxygen species (ROS) production and LAP-mediated pathogen clearance. In vitro, E2 further enhances this process. Since RUBICON stabilizes NOX2 for ROS production, higher E2 levels in women may strengthen macrophage LAP, enhancing sepsis resistance (21).
8. Clinical Implications and Challenges
Understanding role of estrogen in sepsis could inform treatment but presents challenges:
- Therapeutic implementation: Determining optimal dosing and administration.
- Potential risks: Possible thromboembolism and hormonal side effects (22).
- Need for clinical trials: Further research is needed to confirm estrogen’s safety and efficacy.
9. Future Directions
Exploring selective estrogen receptor modulators (SERMs) may offer targeted benefits with fewer side effects.
10. Conclusions
Estrogen’s protective effects in sepsis warrant deeper investigation. Shifting from epidemiological studies to mechanistic insights may unlock new treatment strategies.