1. Background
During cardiac surgeries, the myocardium is highly vulnerable to damage due to numerous factors, leading to different levels of morbidity and mortality (1). The success of a cardiac surgical procedure may be hindered by sudden changes in hemodynamic response, such as arrhythmia, extreme fluctuations in arterial blood pressure and heart rate (HR), global ischemia during cross-clamping in already hypoxic and hypertrophied cardiac muscles, inflammatory cascades developed during cardiopulmonary bypass (CPB), inefficiencies in surgery such as imperfect revascularization during coronary artery bypass graft (CABG), and reperfusion after the operation, which results in the reoxidation of cellular components and cellular necrosis (2). Even with a sufficient depth of anesthesia, CABG surgery involves a variety of painful and intense surgical stimuli that may trigger a stress response (3). Certain triggers, like laryngoscopy, tracheal intubation, skin incisions, and sternotomy, quickly elicit a sympathetic response, leading to an increase in arterial blood pressure and HR (4). Postoperative myocardial injuries are strongly associated with altered blood pressure and HR during cardiac surgery (5).
Dexmedetomidine is a highly selective, centrally acting adrenergic receptor agonist with a short half-life, rendering it a proper medication for intravenous administration to prevent stress responses (4). Dexmedetomidine functions as an anxiolytic, hypnotic, and analgesic agent. As a result, it assists in reducing the hemodynamic response to procedures like laryngoscopy and intubation, enhancing intraoperative hemodynamic stability, and intensifying the effects of general anesthesia. This leads to a reduction in the amount of anesthesia needed for both the induction and maintenance of anesthesia (6). Activation of α2 adrenergic receptors promotes a central depression of sympathetic stimulation that leads to decreased arterial blood pressure and HR, as well as decreased reliance on opioids and more consistent hemodynamics during the initial stages of postoperative recovery (7). Dexmedetomidine also facilitates a safer recovery process and reduces the occurrence of respiratory depression (8).
2. Objectives
The present study aimed to investigate the impact of dexmedetomidine in reducing the hemodynamic response to skin incisions, sternotomy, and the postoperative duration following CABG, on preserving left ventricular function postoperatively while preventing excessive cardiac stunning.
3. Methods
This study was registered as a randomized controlled trial, encompassing 64 patients aged 30 to 60 years of both sexes undergoing elective CABG surgery. The research was conducted following approval from our institutional ethical committee, and written informed consent was obtained from all participants. Exclusion criteria included patients with dementia, significantly impaired ventricular function [ejection fraction (EF) < 40%], uncontrolled diabetes (HbA1c > 8 mg/dL), combined intervention (CABG with valve replacement), urgent surgery, renal impairment (serum creatinine ≥ 2 mg/dL or GFR ≤ 50 mL/min), hepatic impairment (child B or C as per Pugh classification), or off-pump CABG.
Patients were randomly allocated using a computer-generated program into two equal groups (allocation ratio 1:1): Group D received a continuous infusion of dexmedetomidine (0.5 µg/kg/h) starting one hour before the induction of anesthesia and continuing until immediately before transfer to the intensive care unit (ICU), and group C received 0.9% saline infusion at a continuous rate of 10 mL/h as a control group. The night before the surgical procedure, all patients were administered 0.5 mg of alprazolam orally. Subsequently, a pulse oximeter and five-lead electrocardiograms (ECGs) were affixed in the preparation room. Following the administration of local anesthetic, a 20G intra-arterial catheter for invasive blood pressure monitoring was inserted, arterial blood gas (ABG) was obtained on room air, and a triple lumen central venous catheter as well as a wide-bore intravenous cannula (16G) were inserted. All patients received intravenous midazolam for sedation, with a dosage ranging from 0.03 to 0.05 mg/kg. Furthermore, patients in group D were administered a dexmedetomidine infusion instead of a saline infusion, as was the case in the control group.
Following a one-hour period, patients were transferred to the operating room. General anesthesia was initiated using fentanyl (5 - 10 µg/kg; dose-titrated), propofol (1 - 2 mg/kg; response-titrated), and rocuronium (1 mg/kg), leading to endotracheal intubation. Subsequently, the patients were maintained on oxygen/air (FiO2) (40% - 60%), 1.5 - 2.5 vol% end-tidal sevoflurane, and a rocuronium infusion (0.1 - 0.2 mg/kg). A nasopharyngeal temperature probe, transesophageal echocardiography (TEE), and an in-situ urine catheter were inserted. All myocardial preservation procedures were strictly followed during CPB, and a temperature ranging from 30°C to 32°C was maintained. Dexmedetomidine at a dosage of 0.5 µg/kg/h was administered continuously to the study group one hour prior to the induction of anesthesia and was ceased immediately prior to patient transfer to the ICU. In the control group, patients received a continuous infusion of 0.9% saline at a rate of 10 mL/hr. Extubation was performed when patients were fully conscious and exhibited no indications of bleeding or arrhythmias.
The hemodynamic parameters, including MAP and HR, were assessed at T0 (prior to induction), T1 (three minutes after skin incision), T2 (three minutes after sternotomy), T3 (before commencing CPB), T4 (fifteen minutes after CPB discontinuation), T5 (immediately upon arrival in the ICU), and subsequently after four hours in the ICU. Bradycardia, defined as more than a 20% decrease from baseline HR, was managed with atropine 0.5 mg, and a decrease in MAP of < 20% of the baseline MAP was managed by vasopressors (noradrenaline infusion).
Lactic acid levels were measured in the ABG at various time points throughout the patient’s perioperative care: At 0 (baseline), 1 (3 minutes after sternotomy), 2 (prior to weaning from CPB), 3 (15 minutes after CPB), 4 (before transfer to the ICU), 5 (immediately after transfer to the ICU), and finally, after 4 hours in the ICU. The evaluation of ventricular function pre- and post-bypass involved the use of TEE global systolic function EF in M mode to assess for any presence of wall motion abnormality or diastolic function. On the day of the operation, postoperative ventricular function was similarly assessed using transthoracic echocardiography (TTE) global systolic function EF by M mode, with consideration given to wall motion abnormality and diastolic function.
Total narcotics dosage, the maximum inotropic dosage support and mechanical assistance post-surgery, need for pacemaker insertion, instances of prolonged intubation (defined as intubation lasting longer than 24 hours), incidences of post-CPB bleeding and reoperation, occurrence of postoperative arrhythmias such as ventricular fibrillation and atrial fibrillation, length of stay in the ICU, and duration of hospitalization were also recorded. All data were recorded by a physician who was blinded to the drugs administered.
The primary outcome was to assess the impact of dexmedetomidine on mean arterial pressure (MAP) changes during the study period. Secondary outcomes included variations in HR, intraoperative and postoperative ventricular function, serum lactate levels in ABG, duration of stay in the ICU, and length of hospital stay. A sample size of at least 32 cases per group (64 cases) was calculated based on Hashemian et al. (9), who compared MAP and HR between intervention and control groups across 8 points in time and reported a large effect size (> 0.3) within and between treatments. This achieved a power of 80% to determine an efficient size of 0.3 within (9 points of time) as well as between the two groups using repeated-measures ANOVA with an in-between factor with a level of significance of 0.05. A correlation of 0.5 was assumed between the repeated measures, and 20% excess cases were added to compensate for the potential dropout rate (10).
3.1. Statistical Analysis
Statistical analysis was performed using SPSS version 26 (IBM Inc., Chicago, IL, USA). The Shapiro-Wilk test and histograms were used to evaluate the normality of the data distribution. Quantitative variables were presented as mean and standard deviation (SD) and compared between the two groups using the unpaired Student’s t-test. Qualitative variables were presented as frequency and percentage (%) and analyzed using the chi-square test or Fisher’s exact test when appropriate. A two-tailed P-value < 0.05 was considered statistically significant.
4. Results
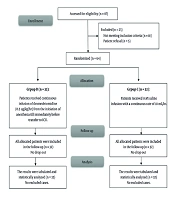
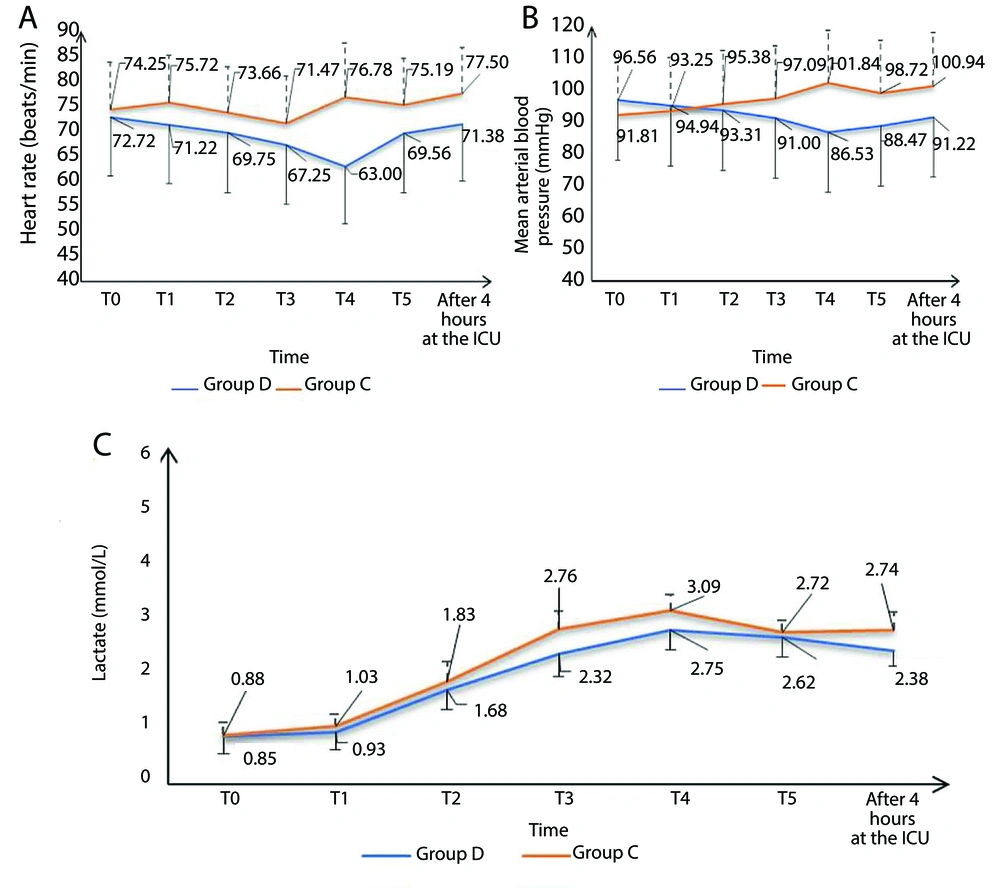
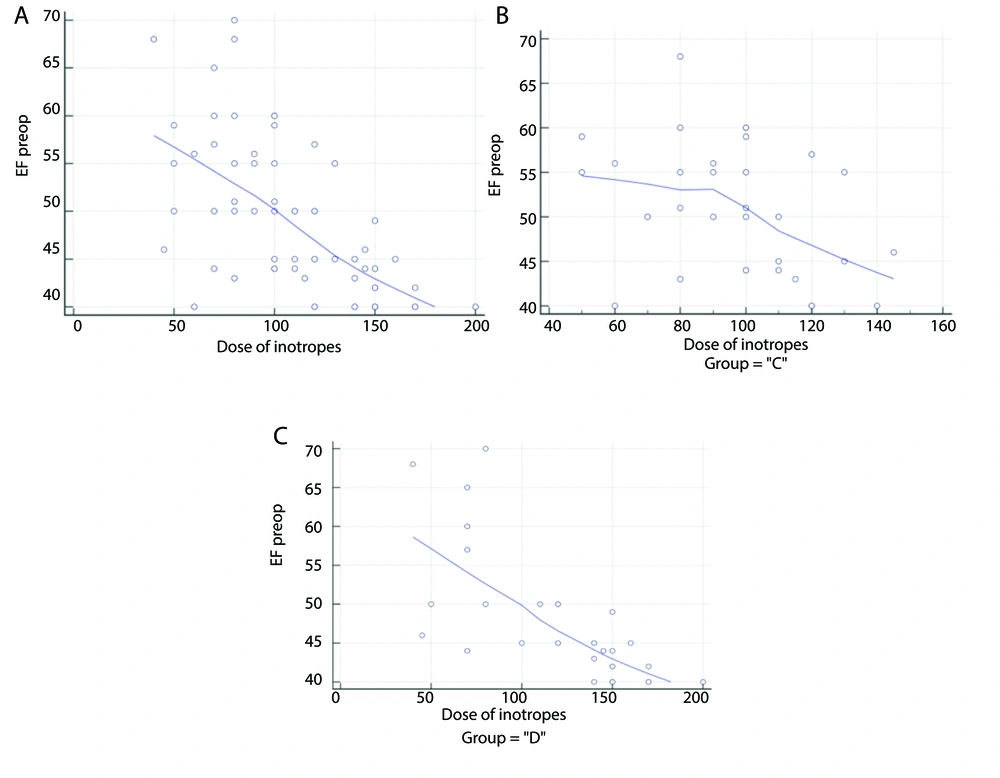
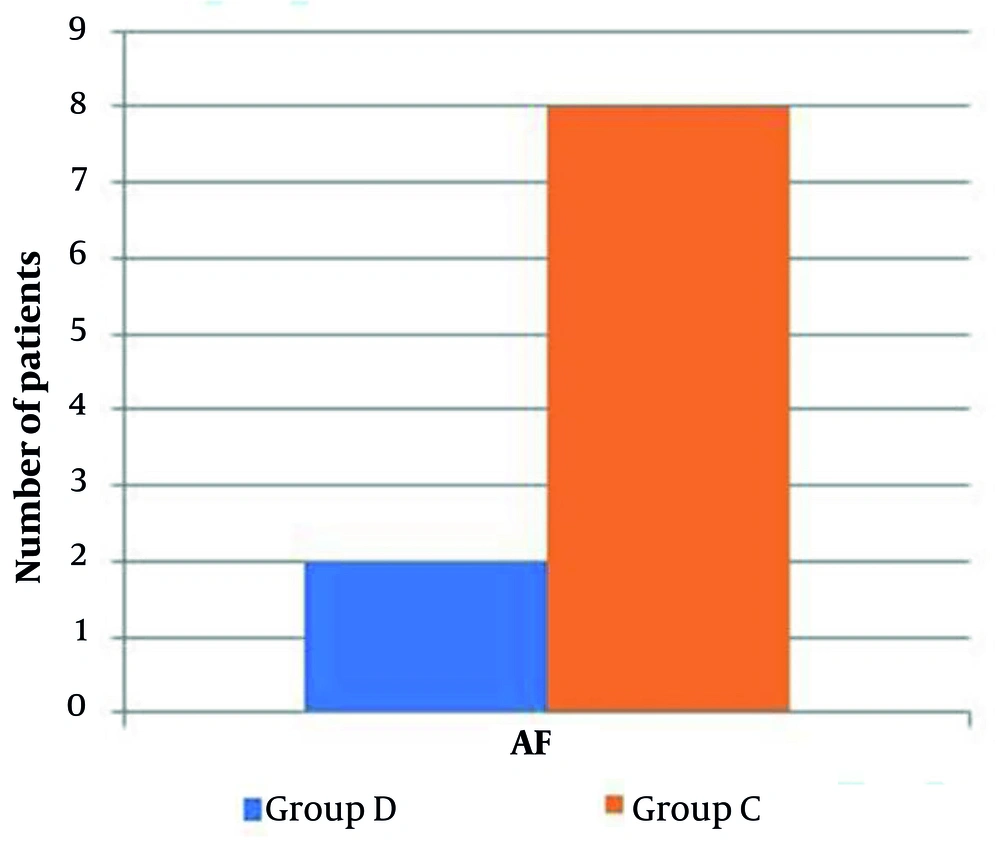
Eighty-seven patients underwent initial evaluation for eligibility. Of these, 18 did not meet the inclusion criteria, and 5 declined to participate. The remaining patients were randomly allocated into two similar groups, with 32 patients each. Subsequently, all enrolled patients were subjected to follow-up and underwent statistical analysis (Figure 1). Age, sex, weight, height, BMI, duration of surgery, and duration of CPB were comparable (Table 1). The HR and MAP exhibited insignificant differences at T0 between both groups but showed significant differences at T1, T2, T3, T4, T5, and after 4 hours in the ICU (P < 0.05). Over time, there were no significant differences in lactate concentration between the groups (Tables 2 , 3, and 4; Figure 2). Although EF was significantly different postoperatively (P = 0.043), it was not significantly different pre- and post-bypass. The incidence of bleeding and reopening was comparable (Table 5). The dosage of inotropes was significantly higher in group D (121.5 ± 41.9) compared to group C (97.18 ± 24.29) (P < 0.003). There was a moderate negative correlation between the EF and the maximum dose of inotropes, with a strong negative correlation in group D and a weak negative correlation in group C (Figure 3A, B, and C). The total dose of narcotics was significantly lower in group D (285 ± 44.9) compared to group C (361.88 ± 60.4) (P < 0.001). In comparing the two groups, it was observed that while there were no significant differences in mechanical ventilation, pacemaker use (Table 6), ventricular fibrillation, or bradycardia, there was a marked decrease in atrial fibrillation incidence in group D (6.25%) compared to group C (25%) (P = 0.04) (Figure 4). The length of ICU stay and hospital stay were significantly shorter in group D (P = 0.0209 and P = 0.0006, respectively) (Table 7).
| Variables | Group D (n = 32) | Group C (n = 32) | P-Value |
|---|---|---|---|
| Age (y) | 48.78 ± 14.82 | 49.75 ± 15.91 | 0.802 |
| Sex | 0.424 | ||
| Male | 23 (71.88) | 20 (62.5) | |
| Female | 9 (28.13) | 12 (37.5) | |
| Weight (kg) | 71.25 ± 11.22 | 72.38 ± 12.08 | 0.701 |
| Height (m) | 1.66 ± 0.08 | 1.68 ± 0.08 | 0.216 |
| BMI (kg/m2) | 26.07 ± 4.95 | 25.62 ± 4.67 | 0.712 |
| Duration of surgery (h) | 4.69 ± 1.71 | 4.88 ± 1.41 | 0.634 |
| Duration of CPB (min) | 62.56 ± 22.14 | 63.81 ± 22.34 | 0.823 |
Abbreviations: BMI, Body Mass Index; CPB, cardiopulmonary bypass.
a Values are expressed as No. (%) or mean ± SD.
b Age, sex, weight, height, BMI, duration of surgery and duration of CPB were insignificantly different between both groups.
| Variables b | Group D (n = 32) | Group C (n = 32) | P-Value |
|---|---|---|---|
| T0 | 72.72 ± 11.69 | 74.25 ± 9.37 | 0.565 |
| T1 | 69.31 ± 12.16 | 75.72 ± 9.42 | 0.022 c |
| T2 | 66.75 ± 12.34 | 75.16 ± 9.72 | 0.004 c |
| T3 | 65.13 ± 12.27 | 74.78 ± 9.93 | 0.001 c |
| T4 | 63.00 ± 11.41 | 76.78 ± 10.82 | < 0.001 c |
| T5 | 69.56 ± 11.82 | 75.19 ± 9.33 | 0.039 c |
| After 4 hours at the ICU | 71.38 ± 11.28 | 77.50 ± 9.17 | 0.020 c |
Abbreviation: ICU, intensive care unit.
a Values are expressed as mean ± SD.
b T0, before induction; T1, 3 minutes following skin incision; T2, 3 minutes following sternotomy; T3, before going on CPB; T4, 15 minutes after weaning from CPB; T5, immediately after transfer to ICU.
c A P-value ≤ 0.05 is considered statistically significant.
| Variables b | Group D (n = 32) | Group C (n = 32) | P-Value |
|---|---|---|---|
| T0 | 96.56 ± 18.82 | 91.81 ± 16.68 | 0.289 |
| T1 | 85.78 ± 16.59 | 94.94 ± 18.86 | 0.043 c |
| T2 | 87.28 ± 19.36 | 98.09 ± 17.46 | 0.022 c |
| T3 | 87.66 ± 19.35 | 97.09 ± 16.55 | 0.040 c |
| T4 | 86.53 ± 18.93 | 101.84 ± 16.58 | 0.001 c |
| T5 | 88.47 ± 18.67 | 98.72 ± 16.54 | 0.023 c |
| After 4 hours at the ICU | 91.22 ± 18.62 | 100.94 ± 16.79 | 0.032 c |
Abbreviation: ICU, intensive care unit.
a Values are expressed as mean ± SD.
b T0, before induction; T1, 3 minutes following skin incision; T2, 3 minutes following sternotomy; T3, before going on CPB; T4, 15 minutes after weaning from CPB; T5, immediately after transfer to ICU.
c A P-value ≤ 0.05 is considered statistically significant.
Abbreviation: ICU, intensive care unit.
a Values are expressed as mean ± SD.
b T0, before induction; T1, 3 minutes following skin incision; T2, 3 minutes following sternotomy; T3, before going on CPB; T4, 15 minutes after weaning from CPB; T5, immediately after transfer to ICU.
c A P-value ≤ 0.05 is considered statistically significant.
| Variables | Group D (n = 32) | Group C (n = 32) | P-Value |
|---|---|---|---|
| Pre bypass | 56.94 ± 9.3 | 54.41 ± 9.6 | 0.289 |
| Post bypass | 60.22 ± 9.1 | 58.38 ± 9.8 | 0.440 |
| Post-surgery | 59.06 ± 7.4 | 54.84 ± 8.9 | 0.043 b |
a Values are expressed as mean ± SD.
b A P-value ≤ 0.05 is considered statistically significant.
Correlation curves between dosage of inotropes and ejection fraction % (EF%). A, moderate negative correlation between dosage o finotropes and EF% among the 2 groups; B, weak negative correlation between dosage of inotropes and EF% among group C; C, strong negartive correlation between dosage of inotropes and EF% among group D.
| Variables | Group D (n = 32) | Group C (n = 32) | P-Value |
|---|---|---|---|
| Dose of inotropes (mic/min) | 6412.5 ± 1010.25 (5040 - 7830) | 4342.5 ± 724.78 (3120 - 5520) | < 0.001 b |
| Dose of narcotics (mic) | 285 ± 44.9 (224 - 348) | 361.88 ± 60.4 (260 - 460) | < 0.001 b |
| Need for pacemaker; No. (%) | 0.554 | ||
| Yes | 1 (3.13) | 2 (6.25) | |
| No | 31 (96.88) | 30 (93.75) | |
| Mechanical ventilation (h) | 25.31 ± 22.54 (4 - 110) | 30.63 ± 24.42 (10 - 122) | 0.369 |
a Values are expressed as mean ± SD (range) unless otherwise indicated.
b A P-value ≤ 0.05 is considered statistically significant.
Abbreviation: ICU, intensive care unit.
a Mann-Whitney test.
b A P-value ≤ 0.05 is considered statistically significant.
5. Discussion
Dexmedetomidine is a selective α2-adrenoceptor agonist widely used as a sedative and analgesic agent in the ICU, as well as an anesthetic adjunct therapy (11). During CPB surgery, dexmedetomidine maintains hemodynamic stability (12). It also reduces the stress response resulting from intubation and surgeries. Owing to its sympatholytic properties, it might reduce O2 consumption by the heart and decrease the HR (13). According to our findings, group D experienced significantly lower HR and MAP at T1, T2, T3, T4, T5, and after 4 hours in the ICU. This may be due to the central inhibitory effect of dexmedetomidine on sympathetic discharge, which decreases the sympathetic tone peripherally in addition to vagal stimulation, leading to a decrease in HR and MAP, which in turn leads to a decrease in myocardial O2 requirements and cardiac afterload, providing cardiac protection, particularly in patients with stenosed coronaries (14).
Significant neuroendocrine reactions are triggered by laryngoscopy and endotracheal intubation, increasing the risk of perioperative ischemia and myocardial infarction (MI). Therefore, when dexmedetomidine is administered preoperatively as a sympatholytic anesthetic adjuvant, it may decrease the stress response and help preserve cardiac perfusion. Dexmedetomidine stabilizes hemodynamic fluctuations during the operation and recovery period by modifying the stress-induced sympathoadrenal reaction to intubation, thus achieving good outcomes. In line with this, a study by Sulaiman et al. (15) reported that dexmedetomidine infusion during surgery reduced the hemodynamic and neuroendocrine responses to trauma caused by surgery and CPB. Consistent with our findings, Kamal et al. (16) concluded that dexmedetomidine significantly reduced the vasoconstriction response to severe surgical stimuli and intubation. Hashemian et al. (9) also showed that dexmedetomidine stabilized ABP and HR during the CPB pump and in the ICU postoperative period. Dexmedetomidine has two effects: It reduces the ABP response to surgical stress and minimizes the rise in ABP and HR following CPB and in the postoperative ICU. However, according to Farsad et al. (17), no significant variation was detected in the hemodynamic values, including HR, MAP, and CVP.
We observed a significant decrease in the total narcotic dosage in group D compared to the control group C. This finding aligns with the results of Gumus (18), who noted that the concurrent use of dexmedetomidine and narcotics can reduce opioid doses during anesthesia induction while also leading to more stable hemodynamic parameters, particularly systolic arterial pressure, in patients undergoing coronary artery bypass grafting. As a result, the incidence of hypertension and fluctuations in arterial pressure may be minimized postoperatively, allowing for more rapid weaning of patients from the ventilator.
In the current study, both groups’ EFs were comparable before and after bypass. However, it was significantly greater on the first postoperative day in group D. Our study’s rationale was that dexmedetomidine reduces the workload on the myocardium, decreasing the incidence of postoperative stunning. Supporting our results, Sulaiman et al. (15) reported that postoperative EF was significantly greater in the dexmedetomidine group than in the control group of CABG patients. In our study, there was no significant difference in lactate concentration over time between the two groups. Similarly, Kamal et al. (16) and Farsad et al. (17) revealed that the levels of lactate and other biochemical parameters were similar in CABG patients in both groups. This highlights that although MAP and HR were significantly lower at some points in group D, they did not affect body perfusion, as lactate was not affected in parallel.
In addition to detecting variations in the need for inotropic support, our study revealed a negative correlation between the patients’ EF% and inotrope dosage. Greater doses of inotropic support were needed for patients with lower EF%. Whereas the control group showed a lesser negative correlation, group D showed a stronger negative correlation, making this correlation especially noticeable. These findings can be explained by the ability of dexmedetomidine to reduce cardiac output by inhibiting HR acceleration while preserving myocardial contractility and stroke volume. As a result, patients with lower EF% were more significantly impacted by the increased dosages of inotropic support. However, they remained weanable, as this adjustment did not adversely affect patient perfusion or lactate levels.
Multiple previous studies suggest that perioperative use of dexmedetomidine may result in a decreased risk of adverse cardiac events, including myocardial ischemia. Alpha-adrenoreceptor stimulation can beneficially modulate coronary blood flow during myocardial ischemia by preventing transmural redistribution of blood flow away from the ischemic endocardium, through specific epicardial vasoconstrictive effects, leading to improvement in endocardial perfusion (the reverse steal effect), and by decreasing HR. This property, along with hemodynamic stability and attenuation of intubation response, makes dexmedetomidine an ideal anesthetic adjuvant, particularly for patients undergoing coronary bypass grafting (19, 20).
According to the current study, there was no statistically significant difference in the need for mechanical ventilation or pacemaker use between the dexmedetomidine and control groups. In contrast to our results, Hu et al. (21) found that dexmedetomidine significantly reduced the time required for mechanical ventilation (18 vs 21 hours). Their results, however, were comparable to ours in terms of the length of stay in the ICU (51 vs 59 hours). This disparity may be explained by the different comparative groups and different sample sizes. Our findings revealed no statistically significant differences in arrhythmia, VF, or bradycardia between both groups. However, the incidence of AF significantly decreased in the dexmedetomidine group. Consistent with our results, Youssef et al. (22) reported no significant differences between the studied groups regarding adverse events, as none of the included patients suffered bradycardia or hypotension, while Liu et al. (23) reported that dexmedetomidine decreased the incidence of novel-onset AF and reduced the duration of ICU stay.
5.1. Limitations
One of the limitations of this study is that it was conducted at a single cardiac surgery center. Moreover, neither pain scores nor plasma norepinephrine levels were measured. Postoperative sedation and extubation reactions were not studied. Additionally, the present study did not examine the QT interval or blood levels of catecholamines, which are more objective ways to assess hemodynamic response to stress and may be used in future studies.
We recommend future studies to conduct the research across multiple cardiac surgery centers to increase the generalizability of the findings. Incorporating standardized pain scoring systems to evaluate postoperative pain levels, which is a significant stressor that influences hemodynamic responses, is also advised. Moreover, measuring plasma norepinephrine levels may provide an objective measure of the body’s stress response. Additionally, measuring the QT interval to assess cardiac repolarization and its relationship to hemodynamic stress may help future studies build on the current findings and contribute to a deeper understanding of hemodynamic stress responses in cardiac surgery patients.