1. Background
Cholelithiasis is a common condition in the general population, which is mostly asymptomatic but may become symptomatic in about 20% of cases. Although cholelithiasis alone is uncomplicated, it causes serious complications in 1 - 2% of cases. Acute cholecystitis, gallstone pancreatitis, and cholangitis are the most common complications of cholelithiasis, which may be life-threatening. Despite the various methods of medical treatments and minimally invasive interventions, the best definitive treatment is still surgery. Every year, more than one million patients are hospitalized because of gallstone complications, and most of them end up undergoing cholecystectomy. In the past, cholecystectomy was performed as an open operation, but for about 30 years, laparoscopic cholecystectomy (LC) has been considered the gold standard of treatment (1-3). Less postoperative pain, smaller incisions, reduced blood loss, shorter hospital stays, and shorter recovery periods are advantages of LC compared to open cholecystectomy (4). Despite all the advantages of laparoscopic surgery, the prevalence of postoperative nausea and vomiting (PONV) in this method is higher than in open surgery (5). The results of studies demonstrated that pneumoperitoneum caused by CO2 during laparoscopy increases the vagal impulse and plays an important role in the occurrence of PONV (6). The PONV is the most common complication after any surgery, but the incidence of PONV after LC is more reported than in other surgeries. 46 - 75% of patients who did not receive antiemetics experienced PONV after LC (7). Despite advances in minimally invasive surgical techniques and anesthesia methods, these symptoms persist (8, 9). Several factors may trigger PONV, such as female gender, volatile and prolonged anesthesia, history of PONV or motion sickness, and non-smokers (10). Severe PONV sometimes can result in aspiration pneumonia, dehydration, electrolyte imbalance, suture dehiscence, and bleeding, which can have serious consequences (10, 11). In recent years, studies have been conducted to find a way to prevent this adverse event. The results of these studies suggest using non-opioid drugs or short-acting analgesics, and less manipulation during gastrointestinal surgery can lower the occurrence of PONV. The current framework for PONV management is based on risk assessment and PONV prophylaxis, but some patients still need rescue treatment. The PONV prophylaxis and rescue treatment include pharmacologic and non-pharmacologic approaches (12, 13). Multiple medications have been used for PONV prophylaxis, such as metoclopramide, ondansetron, dexamethasone, droperidol, and propofol (14-17). Although these drugs are effective when used alone, there is a paradigm shift in PONV management, which is using multiple anti-emetics as a standard of care (18, 19).
5-Hydroxytryptamine type 3 (5HT3) receptor antagonists are the first-line therapy for PONV as they have minor side effects and rarely cause cardiac conduction abnormalities. Ondansetron is a member of this family. It has a relatively short half-life (3 - 5 hours) and may be administered several times a day based on the severity of symptoms (15). Aprepitant is a Neurokinin-1 (NK1) receptor antagonist recently approved for PONV prophylaxis. It has a long half-life with high antiemetic efficacy and few side effects. The NK1 receptors exist in the central nervous system and combine with substance P. Aprepitant can pass the blood-brain barrier and represents high receptor occupancy in a short time. Substance P exists in high concentrations in the vomiting center, where it reacts with the NK1 receptors and is involved in the vomiting reflex in the brain and the stomach. In the literature, there are not many studies on the antiemetic effect of aprepitant in combination with other anti-emetics so far (20-22). Therefore, conducting studies to evaluate its efficacy in combination with other anti-emetics for PONV prophylaxis would be rational, especially when the trend in practice is towards combination therapy (18). Until now, multiple clinical trials have studied the efficacy of aprepitant in combination with ondansetron on PONV, and the combination of these two agents has been effective in reducing PONV in head and neck, gynecological, and plastic surgeries (22-24).
2. Objectives
Considering the high prevalence of gallstones and, as a result, the increased rate of cholecystectomy, the tendency of today's surgeons and patients to perform laparoscopic surgery, the high prevalence of PONV after LC, and the evidence of the effect of the combination of aprepitant and ondansetron on reducing PONV, this study was conducted with the aim of investigating the effect of these two drugs in combination on PONV after LC.
3. Methods
This trial was registered at the Iranian Registry of Clinical Trials (IRCT20221110056459N1, and permission to perform the study was obtained from the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1401.116). All methods in this randomized controlled trial are carried out based on the CONSORT protocol. All participants were informed about the study in detail by one of the authors, and they also signed a written consent form.
3.1. Subjects
This study is a parallel, randomized, double-blinded, placebo-controlled trial. The inclusion criteria were patients who were candidates for LC in a tertiary hospital in Tehran, Iran, between 2021 and 2022, aged 18 to 70 years, and classified as the American Society of Anesthesiologists (ASA) health scale 1 or 2. Patients were excluded if they met any of the following criteria: (1) Patients who refused to sign the consent; (2) patients classified as ASA health scale three, four, or five; (3) patients presented with acute cholecystitis; (4) patients treated with serotonergic agents, ergotamine or erythromycin compounds, apomorphine, and pimozide; (5) pregnant or lactating patients; (6) drug or alcohol abusers and smokers; (7) history of serious hypersensitivity reactions, including anaphylaxis and anaphylactic shock; (8) history of Long QT Syndrome, or presence of prolonged QT in the ECG; (9) patients with any history of motion sickness, digestive problems, psychiatric disorders, and systemic diseases. Randomization was conducted according to random blocks of four. Code A or B was considered for each of the groups, and different blocks of four were made. Then, patients were assigned to different blocks.
3.2. Study Design
First, demographic data and baseline characteristics of all patients were recorded in a checklist, and then patients were randomly assigned to the intervention or control group (group A or B). The intervention group received a capsule (aprepitant 80 mg) one hour before the operation, followed by ondansetron 4 mg administered intravenously at the same time as the operation. The control group was treated in the same way as the intervention group, except that they received a placebo capsule instead of aprepitant. In case of severe nausea after the operation, the patients received an additional dose of ondansetron (maximum every 8 hours). All patients were anesthetized by a single anesthesiologist with propofol. In the recovery and inpatient ward, a single researcher completed the checklist without knowing the type of drugs received and the groups. Patients were assessed for the severity of nausea according to the Likert scale and the presence of vomiting at 6 and 24 hours after the operation. The time to start the examination was exactly when the patient regained consciousness. On this scale, 1 was the least and 10 was the highest amount of nausea. We defined the Likert score 1 - 3 as mild, 4 - 7 as moderate, and 8 - 10 as severe nausea. It should be noted that the patient and the observing researcher/statistical analyst were also blinded. The primary outcome of the study was considered the severity of PONV among the two groups, and the secondary outcomes included investigating the length of hospitalization (LOH) and the impact of basic variables on PONV.
3.3. Statistical Analyses
The data were analyzed using SPSS (IBM, Chicago, IL, USA). Quantitative and qualitative variables were reported as mean ± standard deviation (SD) and frequency and frequency percentage, respectively. The independent samples t-test, Mann–Whitney test, chi-square, and correlation tests were used to assess the postoperative changes of variables and determine associated factors. The significance level was considered P<0.05.
4. Results
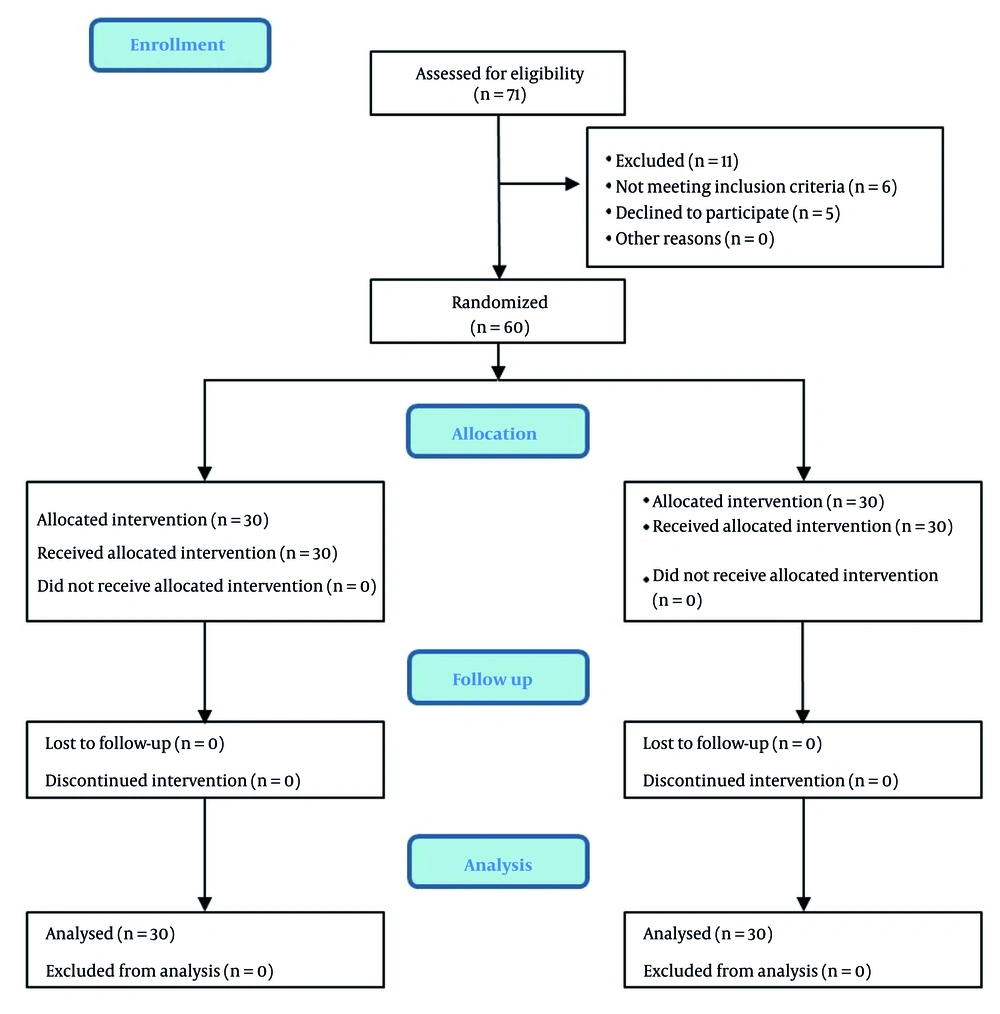
Initially, 71 patients who were candidates for LC were selected for the study. Considering the exclusion criteria, 11 patients were excluded, and finally, 60 patients were included in the study and allocated into two groups equally (Figure 1). Group A was the control group, where patients were administered ondansetron and placebo. Group B was the study group, where patients received ondansetron and aprepitant. To decrease the confounding effect, participants in both groups were matched based on basic characteristics. There was no significant difference between these two groups in terms of age, gender, and Body Mass Index. Also, the results of the study between the two groups showed that the duration of anesthesia and operation did not differ significantly between the two groups (Table 1).
| Variables | Group A Ondansetron + Placebo | Group B Ondansetron + Aprepitant | P-Value |
|---|---|---|---|
| Age (y) | 40.73 ± 11.16 | 39.86 ± 12.29 | 0.96 b |
| Gender | |||
| Male | 10 (33.3) | 10 (33.3) | 1 c |
| Female | 20 (66.4) | 20 (66.4) | |
| Body Mass Index (kg/m2) | 25.4 ± 3.26 | 24.9 ± 3.30 | 0.611 b |
| Duration of anesthesia (min) | 128 ± 22.95 | 123.6 ± 18.65 | 0.503 d |
| Duration of operation (min) | 87.33 ± 20.49 | 85.33 ± 18.33 | 0.69 b |
| PONV [quantitative] 6 h | 6.23 ± 1.45 | 4.46 ± 1.25 | < 0.0001 d |
| PONV [quantitative] 24 h | 3.2 ± 1.39 | 1.33 ± 0.92 | < 0.0001 d |
| PONV [qualitative] 6 h | |||
| Mild | 1 (3.3) | 5 (16.6) | 0.137 c |
| Moderate | 24 (80) | 23 (76.6) | |
| Severe | 5 (16.6) | 2 (6.6) | |
| PONV [qualitative] 24 h | |||
| Mild | 21 (70) | 28 (93.3) | 0.42 c |
| Moderate | 9 (30) | 2 (6.6) | |
| Severe | 0 (0) | 0 (0) | |
| Receiving an additional dose of ondansetron | 10 (33.3) | 2 (6.66) | 0.021 c |
| LOH (d) | 3.2 ± 0.48 | 3.16 ± 0.37 | 0.718 d |
Abbreviation: PONV, postoperative nausea and vomiting; LOH, length of hospitalization.
a Values are expressed No. (%) or mean ± SD.
b Independent t-test.
c Chi-square test.
d Mann-Whitney test.
The mean Likert scale for evaluating nausea at 6 hours after LC was 6.23 ± 1.45 for group A and 4.46 ± 1.25 for group B (P < 0.0001). Besides, at 24 hours after LC, it was 3.20 ± 1.39 for group A and 1.33 ± 0.92 for group B (P < 0.0001). However, when we ranked the severity scale qualitatively, no difference was observed between the two groups. In group A, 10 patients (33.3%) and in group B, 2 patients (6.66%) needed to receive an additional dose of ondansetron, which was significantly less in group B (P = 0.021) (Table 1). There was also a direct correlation between the severity of nausea at 6 and 24 hours after LC (r = 0.821, P < 0.0001).
Moreover, the LOH in group A was 3.2 ± 0.48 days, and in group B, it was 3.16 ± 0.37 days; the difference was not significant (Table 1). The results of the Mann-Whitney test showed that gender has no significant relationship with PONV and LOH. In addition, there were no significant relationships between age, Body Mass Index, duration of anesthesia, and duration of operation with PONV (Table 2).
| Variables | PONV (6 h) | PONV (24 h) | Length of Hospitalization (d) | |||
|---|---|---|---|---|---|---|
| R | P-Value | R | P-Value | R | P-Value | |
| Age (y) | 0.34 | 0.794 | 0.073 | 0.581 | 0.31 | 0.015 |
| Body Mass Index (kg/m2) | 0.12 | 0.32 | 0.17 | 0.17 | 0.24 | 0.058 |
| Duration of anesthesia (min) | 0.211 | 0.105 | 0.113 | 0.391 | -0.184 | 0.159 |
| Duration of operation (min) | 0.148 | 0.258 | 0.079 | 0.551 | -0.022 | 0.91 |
Abbreviation: PONV, postoperative nausea and vomiting; LOH, length of hospitalization.
5. Discussion
The PONV is a significant challenge for surgeons, anesthesiologists, and patients. The prevalence of PONV is estimated at 30%, which may increase to 80% in high-risk patients (6). Management of PONV plays an important role in preventing major complications such as aspiration pneumonia, dehydration, electrolyte imbalance, suture dehiscence, and bleeding (10, 11). Cholecystectomy is a common procedure worldwide. Due to fewer complications, doctors and patients tend to perform this procedure laparoscopically. Despite all the advantages of LC, the incidence of PONV is higher with this method, probably due to the effect of pneumoperitoneum on the vagus nerve (5, 6). Considering the importance of the issues raised, we conducted this trial with the aim of investigating the addition of aprepitant as a newer antiemetic drug to ondansetron in the prevention of postoperative nausea. We chose ondansetron because it is administered as a standard treatment for PONV (15). To eliminate responder bias, one hour prior to surgery, an aprepitant capsule (80 mg) and a placebo capsule were given to the study and control groups, respectively. Both groups received 4 mg ondansetron intravenously at the time of surgery.
This study demonstrated that the combination of aprepitant and ondansetron is more effective on PONV than ondansetron alone. The PONV severity in the study group was significantly lower compared to the control group. However, there was no significant difference in the LOH between the two groups. The direct correlation between PONV at 6- and 24-hours post-operation enables us to predict the severity of PONV at 24 hours post-operation based on the patient's status at 6 hours post-operation. However, the severity of PONV at 24 hours post-surgery is generally lower than its severity at 6 hours post-surgery.
Our results are compatible with earlier studies. Two studies conducted by Sinha et al. (25) and Hassan and Abdelzaam (26) on severely obese patients who underwent laparoscopic bariatric surgery, with a similar treatment protocol to ours, revealed that the addition of aprepitant to ondansetron was more effective than ondansetron alone in lowering the incidence of PONV and delaying the first vomiting episode postoperatively. However, Hassan and Abdelzaam administered dexamethasone intra-operatively to all study groups, which we did not, as we believed it could affect the results (26). These findings were supported by Ham et al. (23), who showed that aprepitant in combination with ondansetron could suppress PONV for up to 24 hours. Also, higher (125 mg) and lower (40 mg) doses of aprepitant were studied by Lim et al. (22) and Vallejo et al. (24), respectively, and they arrived at similar results to other studies. On the other hand, there are studies with different results. In the study by Wajid et al. (27), patients who underwent LC were allocated into two groups and were given 8 mg oral ondansetron or 80 mg aprepitant. Their results demonstrated that although the frequency of PONV in the ondansetron group was higher from 0 - 12 hours postoperatively, its frequency from 12 - 24 hours was higher in the aprepitant group. However, they mentioned that the overall PONV frequency of the ondansetron group was significantly higher compared with the aprepitant group. In addition, some researchers have represented that a single dose of oral aprepitant has a comparable effect with frequent injections of ondansetron up to 24 hours postoperatively. Despite different study protocols and results, we agree with them that the optimal dose for aprepitant should be determined, as some studies with different doses, 40 - 125 mg, provided comparable results (28). Moreover, the cost-effectiveness of using aprepitant must be considered, as it is a relatively expensive drug (20). Several pathways and receptors are involved in triggering nausea and vomiting, such as D2, 5-HT3, NK-1, and others. Furthermore, targeting just one particular receptor would not eliminate PONV completely. This is the reason for the new trend toward administering multiple anti-emetics (18). Thus, aprepitant drug interactions with other anti-emetics should be considered too.
In the end, our study had some limitations. Our sample size was small, and procedures were performed by several surgeons, so these results must be confirmed by further studies with a larger sample size and a single surgeon or at least several surgeons with the same level of experience. Besides, we would benefit from following the patients for a longer time post-surgery to make our results more accurate.
5.1. Conclusions
In conclusion, this study demonstrated that the addition of oral aprepitant to IV ondansetron can reduce the severity of PONV. Since LC is a high-risk procedure for PONV, our results suggest that this treatment regimen could be used in other high-risk patients and conditions as well.