1. Background
Upper abdominal procedures can cause significant morbidity due to discomfort and result in inefficient coughing, which leads to atelectasis. The objectives of perioperative pain treatment are to reduce discomfort, facilitate early mobilization and discharge, and increase patient satisfaction (1). Ultrasound (US)-guided fascial plane blocks have been rapidly incorporated into regional anesthesia practice as an alternative to neuraxial techniques. They involve the injection of local anesthetic (LA) into specific tissue planes to provide analgesia across various anatomic regions (2). A new technique, the ultrasound-guided erector spinae plane (US-ESP) block, targets the ventral and dorsal rami, as well as rami communicants, of the spinal nerves. It has been shown that LA spreads cranially and caudally over many dermatomal levels following injection (3). Additionally, it targets both the ventral and dorsal rami, inhibiting both visceral and somatic pain (4). Following various abdominal, thoracic, breast, and spinal procedures, the US-ESP block was found to provide analgesia in certain prior case studies and clinical randomized controlled trials (3).
The external oblique intercostal plane (EOIP) block is a recently described fascial plane block that covers the anterior and lateral upper abdominal wall (5). It covers the lateral and anterior sensory branches of the intercostal nerves from T6 to T11, offering a unique approach for anterolateral upper abdominal wall pain management. It has the advantage of being easier to apply because it is more superficial than transversus abdominis plane (TAP), erector spinae plane (ESP), and quadratus lumborum (QL) blocks. This is especially beneficial for individuals who are obese. Because it is far from vascular structures and the catheter insertion location is far from the procedure site, it can also be done in the supine position (6). In addition to its analgesic potential, the EOIP block's remote access point from vascular structures offers a much lower risk when employed in anticoagulated individuals. Its superficial approach also makes it preferable to traditional regional techniques for obese patients. For patients experiencing acute pain, it is advantageous to have the option to place a catheter away from the surgical site without requiring specific patient posture (7).
2. Objectives
To investigate the effectiveness of the US-guided EOIP block compared to the US-ESP block for pain relief following upper abdominal procedures.
3. Methods
This randomized controlled clinical study received approval from our institute's Ethical Committee of Scientific Research at the Faculty of Medicine, Ain Shams University Hospital, Cairo, Egypt (FAMSU MD80/2023). It was registered with Clinical Trials (registration No NCT06097286). We obtained written informed consent from 75 patients, aged 21 to 60, who were undergoing upper abdominal surgeries and had physical status I-I based on their American Society of Anesthesiologists (ASA) score. Exclusion criteria included those who declined to participate, were younger than 20 or older than 60, or had physical status III-IV according to ASA with a history of coagulopathy and bleeding disorders (INR > 1.6 and PTT > 50 sec), a LA allergy, pre-existing myopathy or neuropathy, an injection site infection, a history of chronic pain syndromes, or a history of long-term opioid or steroid use prior to surgery.
A nurse randomly selected the envelope indicating the assigned group using computer-generated random numbers concealed in opaque, sealed envelopes. Seventy-five patients were randomly assigned into three equal groups of 25 patients each. This study followed a parallel trial design, utilizing block randomization. Five blocks were used to randomize the 75 cases with an allocation ratio of 1:1:1. Each block contained 5 patients per group, totaling 15 patients per block and 75 across all five blocks. The EOIP group received a US-guided EOIP block. The ESP group received a US-ESP block. The Control group did not receive any blocks and received postoperative IV analgesia according to hospital protocol (morphine 0.1 mg/kg). All blocks were administered by an experienced anesthesiologist. Group allocation was blinded to anesthesia residents, surgeons, and patients. The anesthesia residents, responsible for patient follow-up and data collection, were not involved in any other aspects of the study or the drugs used.
3.1. Sample Size
Assuming an effect size difference of 0.4 across groups with respect to post-operative Visual Analog Scale (VAS) score and after a 5% correction for dropout rate, the sample size was calculated using the PASS 15 program. Using an F test with a 0.0500 significance level, the entire sample of 75 patients (25 in each group) provides 82% power to detect differences between the means against the alternative of equal means. The effect size f = om / o, or 0.4000, indicates the magnitude of the mean variation. Based on the findings of prior relevant studies [Malawat et al. (4) and Elsharkawy et al. (2)], an expected effect size of 0.40 was assumed to detect differences in postoperative VAS scores among the three study groups.
Preoperative evaluations included a comprehensive history, clinical examination, regular laboratory tests including complete blood count, liver function test, kidney function test, prothrombin time, and partial thromboplastin time, as well as routine investigations including electrocardiography (ECG) and chest X-ray. All selected patients were informed in detail about the purpose of this study, the procedure, and the potential adverse effects. Written informed consent was obtained.
Inside the operating theatre, intravenous access was secured, standard monitoring was applied, and all procedures were conducted under complete aseptic conditions. Baseline mean arterial blood pressure (MAP), heart rate (HR), ECG, and oxygen saturation (SpO2) were recorded. Intravenous propofol 2 mg/kg, atracurium 0.5 mg/kg, and fentanyl 2 μg/kg were used for induction and analgesia. After relaxation, an endotracheal tube of standard size (appropriate for the patient) was used for intubation. Anesthesia was maintained with a 50:50 oxygen to air mixture, 1 vol% to 1.5 vol% isoflurane, and mechanical ventilation to achieve an end-tidal CO2 of approximately 35 mmHg. Throughout the procedure, intraoperative monitoring of the capnograph, ECG, HR, MAP, and SpO2 was maintained.
In the EOIP Group, a high-frequency linear probe of the Fugi film-Sonosite® M-Turbo US system was positioned at the anterior axillary line at the sixth intercostal space in a longitudinal parasagittal orientation, following surgery and prior to the end of anesthesia. Using an in-plane technique, a 21G 10 cm needle was inserted. The needle tip was advanced into the fascial plane on the deep aspect of the external oblique muscle. A total of 20 mL of the LA mixture (10 mL of bupivacaine 0.5%, 5 mL of lidocaine 2%, and 5 mL of normal saline) was administered. For the other side (if necessary), the same process was repeated.
In the ESP Group, a high-frequency linear probe of the Fugi film-Sonosite® M-Turbo US system was positioned 2.5 - 3 cm laterally to the T9 spinous process in a longitudinal parasagittal orientation, following surgery and prior to the end of anesthesia. The muscles of the erector spinae were found to be superficial to the T9 transverse process tip. Using an in-plane technique, a 21G 10 cm needle was inserted. The needle tip was advanced into the fascial plane on the deep aspect of the erector spinae muscle. On US imaging, the erector spinae muscle was lifted off the bone silhouette of the transverse process by a visible fluid spread, confirming the needle tip's position. A total of 20 mL of the LA mixture (10 mL of bupivacaine 0.5%, 5 mL of lidocaine 2%, and 5 mL of normal saline) was administered. For the other side (if necessary), the same process was repeated.
In the Control Group, patients did not receive any blocks and received postoperative IV analgesia according to hospital protocol (morphine 0.1 mg/kg). Postoperative hemodynamic parameters, including HR and MAP, were recorded immediately at PACU admission and at 2, 4, 6, 8, 12, 18, and 24 hours. All patients received IV paracetamol (10 - 15 mg/kg) every 8 hours for postoperative pain management. Postoperative pain and opioid consumption were assessed using the VAS. Patients with VAS > 3 received IV pethidine (25 - 50 mg) as a second rescue analgesia until VAS became ≤ 3. Postoperative adverse effects, such as nausea and vomiting, were recorded and treated. The time to start mobilization was also recorded.
The study’s primary outcome was the total 24-hour pethidine intake after surgery. The secondary outcomes included the rate of requesting pethidine, duration of technique, ease of technique, VAS scores at rest and during movement, HR, MAP, occurrence of postoperative complications such as nausea and vomiting, and the time to start mobilization. The endpoint was 24 hours postoperatively.
4. Results
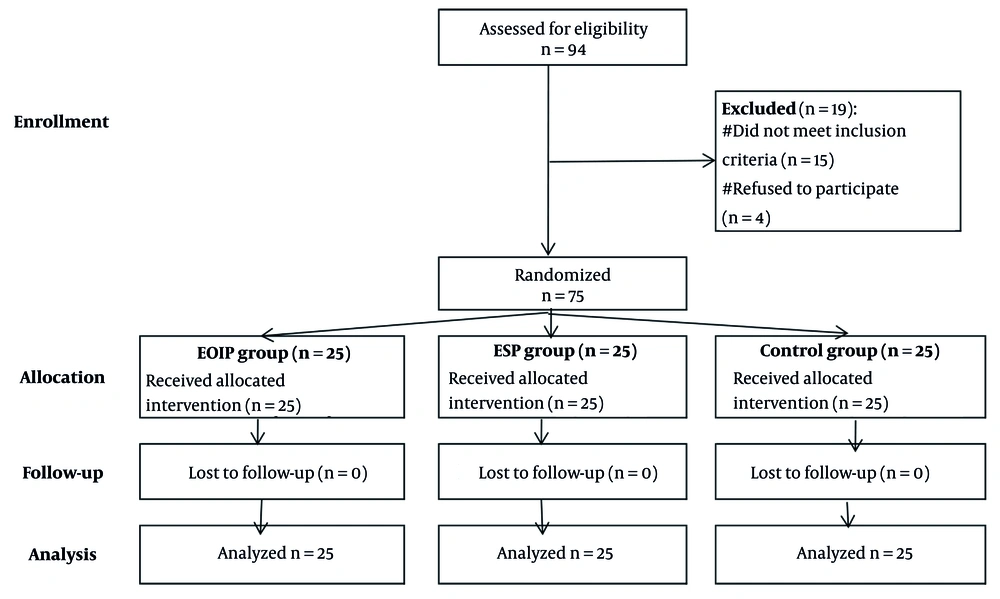
This study divided 75 eligible participants into three groups (EOIP, ESP, and control) after excluding 19 individuals. All participants completed the study with no dropouts, ensuring robust analysis (n = 25 per group). The design minimizes bias and supports reliable comparison of outcomes (Figure 1).
There were no statistically significant differences between the studied groups regarding age, sex, BMI, ASA grade, type of surgery, and operation duration (Table 1).
| Variables | EOIP Group (Total = 25) | ESP Group (Total = 25) | Control Group (Total = 25) | P-Value |
|---|---|---|---|---|
| Age (y) | 44.8 ± 10.4 | 46.9 ± 10.2 | 49.1 ± 8.4 | 0.302 |
| Sex | 0.687 | |||
| Male | 13 (52.0) | 12 (48.0) | 15 (60.0) | |
| Female | 12 (48.0) | 13 (52.0) | 10 (40.0) | |
| BMI (kg/m2) | 28.2 ± 2.8 | 28.8 ± 3.2 | 29.2 ± 3.0 | 0.461 |
| ASA | 0.948 | |||
| I | 13 (52.0) | 12 (48.0) | 13 (52.0) | |
| II | 12 (48.0) | 13 (52.0) | 12 (48.0) | |
| Type of surgery | 0.999 | |||
| Bile duct exploration | 6 (24.0) | 6 (24.0) | 5 (20.0) | |
| Distal pancreatectomy | 3 (12.0) | 4 (16.0) | 4 (16.0) | |
| Hepatectomy | 7 (28.0) | 5 (20.0) | 5 (20.0) | |
| Splenectomy | 4 (16.0) | 5 (20.0) | 5 (20.0) | |
| Whipple | 5 (20.0) | 5 (20.0) | 6 (24.0) | |
| Operation duration (h) | 3.9 ± 0.5 | 3.7 ± 0.6 | 3.6 ± 0.7 | 0.273 |
Abbreviations: EOIP, external oblique intercostal plane; ESP, erector spinae plane; BMI, Body Mass Index; ASA, American Society of Anesthesiologists.
a Values are expressed as mean ± SD or No. (%).
Postoperative HR and mean arterial pressure at follow-up time periods from hour-0 to hour-12 were significantly highest in the control group, with no significant differences between the EOIP and ESP groups (Table 2).
| Variables | EOIP Group (Total = 25) | ESP Group (Total = 25) | Control Group (Total = 25) | P-Value |
|---|---|---|---|---|
| Postoperative heart rate (beat/min) | ||||
| Hour-0 | 75.4 ± 8.1 A | 74.6 ± 8.2 A | 82.2 ± 9.2 B | 0.004 |
| Hour-2 | 76.5 ± 8.6 A | 75.4 ± 8.5 A | 83.2 ± 9.2 B | 0.005 |
| Hour-4 | 78.1 ± 8.0 A | 76.7 ± 7.6 A | 85.7 ± 10.7 B | 0.001 |
| Hour-6 | 80.0 ± 8.3 A | 78.8 ± 8.2 A | 86.2 ± 9.2 B | 0.007 |
| Hour-8 | 80.5 ± 8.1 A | 79.2 ± 7.8 A | 87.5 ± 9.8 B | 0.002 |
| Hour-12 | 83.9 ± 7.7 A | 82.7 ± 7.5 A | 90.2 ± 9.5 B | 0.004 |
| Hour-18 | 81.7 ± 7.7 | 81.3 ± 7.4 | 82.4 ± 8.8 | 0.884 |
| Hour-24 | 81.2 ± 8.3 | 80.5 ± 7.8 | 81.8 ± 9.0 | 0.848 |
| Postoperative mean arterial pressure (mmHg) | ||||
| Hour-0 | 90.2 ± 9.9 A | 89.7 ± 10.3 A | 99.2 ± 11.6 B | 0.003 |
| Hour-2 | 92.5 ± 11.4 A | 90.3 ± 10.6 A | 100.2 ± 11.3 B | 0.006 |
| Hour-4 | 93.4 ± 10.1 A | 92.8 ± 11.0 A | 102.8 ± 12.5 B | 0.003 |
| Hour-6 | 95.9 ± 9.7 A | 94.6 ± 10.4 A | 102.8 ± 9.7 B | 0.009 |
| Hour-8 | 96.6 ± 10.6 A | 95.1 ± 10.9 A | 104.9 ± 10.8 B | 0.004 |
| Hour-12 | 98.2 ± 8.4 A | 97.2 ± 8.4 A | 105.0 ± 9.8 B | 0.005 |
| Hour-18 | 97.4 ± 9.5 | 97.5 ± 8.8 | 99.2 ± 10.6 | 0.755 |
| Hour-24 | 96.9 ± 10.9 | 96.7 ± 9.8 | 97.8 ± 11.0 | 0.922 |
Abbreviations: EOIP, external oblique intercostal plane; ESP, erector spinae plane.
a Values are expressed as mean ± SD.
b Groups that had the same capital superscripted letters (A, B) are Homogenous based on pot hoc Bonferroni test.
Postoperative pain scores, both at rest and during movement, were significantly highest in the control group at follow-up time points from hour-0 to hour-12. No significant differences were noted between the EOIP and ESP groups (Table 3).
| Variables | EOIP Group (Total = 25) | ESP Group (Total = 25) | Control Group (Total = 25) | P-Value |
|---|---|---|---|---|
| Pain score (VAS-10) at rest | ||||
| Hour-0 | 0.8 ± 0.4 A | 0.6 ± 0.5 A | 2.0 ± 0.1 B | < 0.001 |
| Hour-2 | 1.5 ± 0.5 A | 1.2 ± 0.4 A | 3.0 ± 0.6 B | < 0.001 |
| Hour-4 | 2.6 ± 0.5 A | 2.3 ± 0.5 A | 3.3 ± 0.7 B | < 0.001 |
| Hour-6 | 3.2 ± 0.6 A | 2.9 ± 0.6 A | 3.7 ± 0.8 B | < 0.001 |
| Hour-8 | 3.4 ± 0.6 A | 3.2 ± 0.4 A | 4.3 ± 0.5 B | < 0.001 |
| Hour-12 | 3.1 ± 0.7 A | 2.9 ± 0.7 A | 3.6 ± 0.5 B | 0.001 |
| Hour-18 | 3.0 ± 0.6 | 2.8 ± 0.6 | 3.2 ± 0.4 | 0.092 |
| Hour-24 | 2.6 ± 0.5 | 2.4 ± 0.5 | 2.8 ± 0.7 | 0.158 |
| Pain score (VAS-10) on movement | ||||
| Hour-0 | 1.6 ± 0.6 A | 1.6 ± 0.7 A | 2.9 ± 0.6 B | < 0.001 |
| Hour-2 | 2.1 ± 0.6 A | 2.1 ± 0.7 A | 3.9 ± 0.9 B | < 0.001 |
| Hour-4 | 3.5 ± 0.9 A | 3.3 ± 0.7 A | 4.4 ± 0.8 B | < 0.001 |
| Hour-6 | 4.2 ± 0.6 A | 3.8 ± 0.7 A | 4.7 ± 0.8 B | < 0.001 |
| Hour-8 | 4.3 ± 0.8 A | 4.1 ± 0.8 A | 5.3 ± 0.7 B | < 0.001 |
| Hour-12 | 4.0 ± 0.8 A | 3.7 ± 0.8 A | 4.5 ± 0.6 B | 0.002 |
| Hour-18 | 4.0 ± 0.8 | 3.7 ± 0.6 | 3.9 ± 0.6 | 0.397 |
| Hour-24 | 3.6 ± 0.9 | 3.4 ± 0.6 | 3.7 ± 0.9 | 0.296 |
Abbreviations: EOIP, external oblique intercostal plane; ESP, erector spinae plane; VAS, Visual Analog Scale.
a Values are expressed as mean ± SD.
b Groups that had the same capital superscripted letters (A, B) are Homogenous based on pot hoc Bonferroni test.
The time to the first pethidine dose was significantly shortest in the control group, with no significant differences between the EOIP and ESP groups. The total 24-hour pethidine dose was significantly highest in the control group, with no significant differences between the EOIP and ESP groups. The time to mobilization was significantly longest in the control group, with no significant differences between the EOIP and ESP groups (Table 4).
| Variables | EOIP Group (Total = 25) | ESP Group (Total = 25) | Control Group (Total = 25) | P-Value |
|---|---|---|---|---|
| Time to first dose (h) | 8.3 ± 1.6 | 9.2 ± 1.5 | 3.5 ± 1.8 B | < 0.001 |
| Total 24-hour dose (mg) | 33.0 ± 13.9 A | 26.0 ± 5.0 A | 74.0 ± 24.5 B | < 0.001 |
| Time to mobilization (h) | 3.7 ± 0.6 A | 3.4 ± 0.6 A | 5.3 ± 0.7 B | < 0.001 |
Abbreviations: EOIP, external oblique intercostal plane; ESP, erector spinae plane.
a Values are expressed as mean ± SD.
b Groups that had the same capital superscripted letters (A, B) are Homogenous based on pot hoc Bonferroni test.
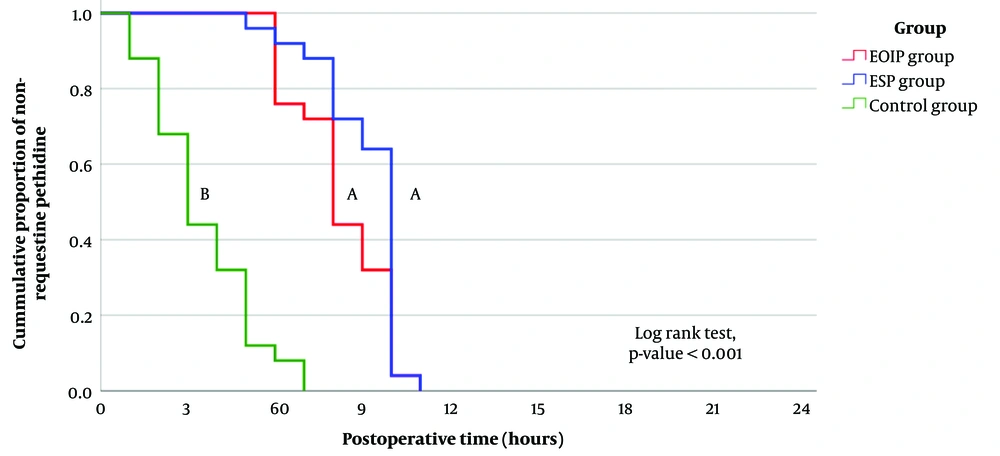
The rate of requesting pethidine was significantly highest in the control group, with no significant differences between the EOIP and ESP groups (Figure 2).
Postoperative nausea and vomiting were most frequent in the control group, with no significant differences between the EOIP and ESP groups, although the differences were significant only for nausea (Table 5).
| Side Effects | EOIP Group (Total = 25) | ESP Group (Total = 25) | Control Group (Total = 25) | P-Value |
|---|---|---|---|---|
| Nausea | 1 (4.0) A | 1 (4.0) A | 8 (32.0) B | 0.005 |
| Vomiting | 0 (0.0) | 0 (0.0) | 1 (4.0) | 0.999 |
Abbreviations: EOIP, external oblique intercostal plane; ESP, erector spinae plane.
a Values are expressed as No. (%).
b Groups that had the same capital superscripted letters (A, B) are Homogenous based on pot hoc Bonferroni test.
The duration of the technique was significantly shorter in the EOIP group. According to the operator’s opinion, the ease of technique score was significantly higher in the EOIP group than in the ESP group (with a score of 0 indicating no satisfaction and a score of 10 indicating maximum satisfaction) (Table 6).
| Variables | EOIP Group (Total = 25) | ESP Group (Total = 25) | P-Value |
|---|---|---|---|
| Duration of technique (min.) | 5.9 ± 1.4 | 11.2 ± 1.2 | < 0.001 |
| Ease of technique (scale-10) | 6.2 ± 1.7 | 4.7 ± 1.7 | 0.004 |
Abbreviations: EOIP, external oblique intercostal plane; ESP, erector spinae plane.
a Values are expressed as mean ± SD.
In conclusion, both the EOIP block and ESP block provide efficient analgesia for upper abdominal surgeries; however, the EOIP block has the advantage of being easier to perform and requiring less time.
5. Discussion
Upper abdominal incisions cause severe intraoperative and postoperative pain. While neuraxial techniques remain the gold standard for pain management, they can be associated with complications. Interfascial plane block techniques have been identified as a part of multimodal analgesia for upper abdominal surgeries involving subcostal incisions (8). The current study showed that postoperative pain scores at rest from hour-0 to hour-12 were significantly highest in the control group with a P-value < 0.001, while there were no significant differences between the EOIP and ESP groups across all intervals (P < 0.05).
Kavakli et al. (6) conducted a randomized study comparing EOIP block and control groups in patients undergoing sleeve gastrectomy performed laparoscopically. Their findings showed significantly lower pain scores at rest and during movement in the EOIP group at 12 hours postoperatively, along with reduced morphine consumption within 24 hours. This is consistent with Helwa et al. (9), who reported that the EOIP block group showed a significantly lower VAS score compared to the control group at 0 time, 1h, 2h, 4h, 8h, and 12h postoperatively with a P-value < 0.001, but no significant difference was observed at 24h.
This study showed that the time to the first pethidine dose was significantly shortest in the control group, with no significant differences between the EOIP and ESP groups. The total 24-hour pethidine dose was significantly highest in the control group, with no significant differences between the EOIP and ESP groups. These findings are consistent with those of Helwa et al. (9), who found that the first call for rescue analgesia was significantly longer in the EOIP group in hours, while the postoperative 24-hour morphine consumption was significantly lower in the EOIP group than in the control group.
In contrast, the study by Samtani et al. (10), which involved 30 patients randomly assigned to two groups for a comparative analysis of analgesic effectiveness between the EOIP and ESP blocks in laparoscopic cholecystectomy, found that the EOIP block provided effective analgesia and reduced opioid requirements. Their results indicated benefits such as technical simplicity and a shorter time to perform the block in the EOIP group compared to the ESP group.
While this study showed no significant differences in analgesic outcomes between the EOIP and ESP groups, Samtani et al. (10) reported that the EOIP block was superior in terms of reducing opioid use and technical performance. This discrepancy highlights the need for further investigation into the factors influencing analgesic effectiveness and the potential variability in outcomes based on different surgical contexts or patient populations.
This study revealed that the time to mobilization was significantly longer in the control group compared to the EOIP and ESP groups, with no statistically significant difference between the EOIP and ESP groups regarding time to mobilization. This finding is consistent with Ozdemir et al. (11), who compared the efficacy of the bilateral erector spinae plane block (ESPB) and the subcostal transversus abdominis plane block (STAPB) under US guidance. They demonstrated that in the STABP group, the time to achieve unassisted walking was longer compared to the ESBP group.
This study reported that nausea and vomiting were most frequent in the control group, with no significant differences between the EOIP and ESP groups, although the differences were significant only for nausea. This is in agreement with Helwa et al. (9), who reported a statistically significant difference between the two studied groups regarding postoperative nausea and vomiting.
In this study, we found that the duration of the technique was significantly shorter in the EOIP group. According to the operator’s opinion, the ease of technique score was significantly higher in the EOIP group than in the ESP group. This finding aligns with the study by Samtani et al. (10), which involved 30 patients randomly assigned to two groups for a comparative analysis of analgesic effectiveness between EOIP and ESP blocks in laparoscopic cholecystectomy. They found that the EOIP block provided effective analgesia and reduced opioid requirements. Their results indicated benefits such as technical simplicity and a shorter time to perform the block in the EOIP group compared to the ESP group.
White and Ji (12) reported that the use of traditional neuraxial procedures has numerous drawbacks, especially when dealing with obese individuals. These include technical challenges related to the anatomical target site's depth, proximity to the operating field, the toxicity of LAs, and contraindications related to infection or coagulation. However, there were no recorded complications or harm in this study as it was US-guided. Complications related to narcotics administration include hypotension, nausea, vomiting, constipation, urinary retention, and itching, and this study recorded only nausea and vomiting.
5.1. Conclusions
Both the EOIP block and ESP block provide efficient analgesia for upper surgical procedures on the abdomen; however, the EOIP block has the advantage of being easier to perform and requiring less time. Future research with larger sample sizes and investigations into the optimal dosages and concentrations of LAs is recommended.
5.2. Limitations
Although the sample size was calculated in our study, the number of included patients was relatively small, and the findings should be validated by future studies with larger sample sizes. There are currently no optimal concentrations and dosages for LAs; this may be the subject of future research. There were no detected sources of bias.