1. Background
Fractures involving the hip region represent a prevalent clinical concern across diverse age demographics, frequently manifesting with severe pain and substantial functional limitation. In geriatric populations, such injuries constitute a critical orthopedic emergency due to their high association with perioperative morbidity, extended hospitalization, and elevated mortality risk, thereby posing a significant burden on healthcare systems (1). Hip surgeries are among the frequently performed procedures in orthopedic surgery. Typically, these surgeries are conducted using subarachnoid blocks, which makes managing postoperative pain difficult (2). Despite their historical prominence as the primary modality for perioperative analgesia, opioid-based regimens are increasingly scrutinized due to their well-documented association with a spectrum of complications encompassing, though not confined to, respiratory compromise, gastrointestinal dysfunction, and opioid-induced hyperalgesia. This growing concern has catalyzed a paradigm shift toward the utilization of regional anesthetic strategies, particularly peripheral nerve blocks, which offer a targeted, opioid-sparing approach capable of attenuating nociceptive transmission in both the preoperative and postoperative phases of surgical care (3). Effective management of pain after surgery and prompt recovery are crucial for achieving positive results following hip surgeries. In previous practices, fascia iliaca compartment blocks (FICBs), lumbar epidurals, and femoral blocks have been utilized to deliver pain relief after operations (3). The integration of ultrasonography into anesthetic practice has revolutionized regional techniques by allowing real-time identification of nerves, accurate needle placement, and monitoring of anesthetic dispersion, collectively contributing to enhanced therapeutic performance and risk-benefit profile of peripheral nerve blockade techniques (4).
The PENG block, initially delineated and characterized by Girón-Arango et al., represents a new regional analgesia method aimed at alleviating pain following total hip arthroplasties (THAs) without affecting motor function. This approach involves administering the local anesthetic within the fascial plane located between the superior pubic ramus and the psoas muscle (4).
Dexmedetomidine is a pharmacodynamic agent characterized by its exceptional affinity and specificity for alpha-2 adrenergic receptor subtypes, through which it orchestrates a constellation of central sympatholytic, analgesic, and sedative effects via presynaptic inhibition of norepinephrine release within the locus coeruleus, making it useful in multimodal analgesia (5).
Steroids possess strong anti-inflammatory and pain-relieving properties. They reduce inflammation by inhibiting phospholipase A2. Dexamethasone is a highly effective and selectively potent glucocorticoid (6).
Although several studies have compared dexmedetomidine and dexamethasone as adjuvants in peripheral nerve blocks, many have methodological limitations such as variability in block types, inconsistent dosing protocols, the usage of additional agents, and the addition of multimodal analgesia regimens, potentially minimizing the observable differences in opioid consumption, which may confound the analgesic outcomes (7).
2. Objectives
To evaluate and compare the analgesic effectiveness of bupivacaine combined with either dexmedetomidine or dexamethasone when administered via PENG block for postoperative pain control in patients undergoing hip surgery.
3. Methods
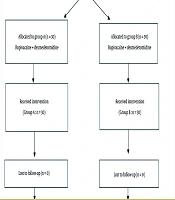
This study was designed as a prospective interventional study and conducted at Ain Shams University hospitals, Faculty of Medicine, Ain Shams University. Scientific and ethical approval was obtained from the Faculty of Medicine, Ain Shams University Research Ethics Committee (FMASUREC) on May 5, 2023, with the approval number MD 113/2023, and Clinical Trial registration number: NCT06294665. Informed consent was obtained from all participants prior to enrollment. Consent was taken directly when the patient was cognitively intact and able to understand the study details. In cases where the patient was unable to provide consent due to cognitive impairment or being in severe pain at the time of enrollment, consent was obtained from a legally authorized representative (a first-degree relative or guardian), in accordance with the institutional ethics committee guidelines.
3.1. Sample Size
Based on the results of Abdelnaiem et al. 2018, with the mean time till first analgesia intervention group being 160 minutes compared to 60 minutes in the control group, an alpha error of 5%, and a power of study of 80%, the required sample size is 60 patients, 30 in each group (5). Eligible candidates were both male and female adults aged from 18 up to 65 years, with an American Society of Anesthesiologists (ASA) classification score ranging from 1 to 2, and prospectively enrolled for elective operative intervention addressing hip joint pathology under spinal anesthesia. Excluded were patients who declined to participate, those who were allergic to the medications used in the study, those who had a psychiatric disorder, patients who had any contraindications to regional anesthesia such as bleeding disorders or local infection at the injection site, patients classified as ASA 3 and 4, and those with prior hip surgery on the affected side.
Patients were randomized using a computer-generated block randomization list. The study was double-blinded: Both patients and outcome assessors were unaware of group allocations. Syringes were prepared by an independent anesthesiologist not involved in data collection. A preoperative evaluation, including a full medical history, examination, and laboratory and imaging investigations, was conducted. Throughout the intraoperative phase, meticulous hemodynamic surveillance was ensured to promptly detect any physiological deviations. A large-bore intravenous cannula was inserted, and lactated Ringer’s solution was administered as a 500 mL bolus given as a preload before performing spinal anesthesia. Spinal anesthesia was performed by an anesthesiologist with more than five years of experience post-qualification in all patients using a twenty-five-gauge spinal needle, through which 15 mg of hyperbaric 0.5% bupivacaine was administered intrathecally. The level of sensory block following spinal anesthesia was assessed using pinprick testing five minutes after intrathecal injection, and surgery was initiated once a T10 level or higher was confirmed in all patients to ensure uniformity in intraoperative analgesia.
Upon completion of the surgical procedure, the PENG block was administered by another anesthesiologist with more than five years of experience post-qualification, with the patient positioned supine and the procedural leg slightly abducted. An ultrasound probe (curvilinear, 2 - 5 MHz; C60xp, SonoSite X-Porte, USA) was applied parallel to the inguinal ligament and then rotated 45 degrees to visualize key landmarks, including the anterior inferior iliac spine, iliopubic eminence, and the psoas tendon. Under ultrasound guidance, an 80 mm, 22-gauge echogenic needle was advanced using an in-plane approach to target the fascial plane between the pubic ramus and the psoas tendon. Following negative aspiration, 20 mL of drug solution was injected from one of the pre-numbered syringes, according to random group allocation.
Patients were randomized using computer-generated numbers into two groups: Group A received a combination of 0.25% bupivacaine with dexmedetomidine (1 μg/kg), while group B received 0.25% bupivacaine with dexamethasone (8 mg); both with a total volume of 20 mL. The Numerical Rating Scale (NRS) of pain was assessed by a third blinded observer who was unaware of the group allocation starting 30 minutes after the patient's admission to the Post-Anesthesia Care Unit, and then at 4-hour intervals over a 24-hour period.
1. Primary outcome of this study: Time for rescue analgesia, with rescue administered at an NRS score ≥ 4 by giving Nalbuphine (5 mg/slowly 4) as a first dose.
2. Secondary outcomes of this study: Cumulative administered dose of nalbuphine within the first 24 postoperative hours, and the occurrence of complications such as hypotension, bradycardia, nausea, and vomiting.
4. Results
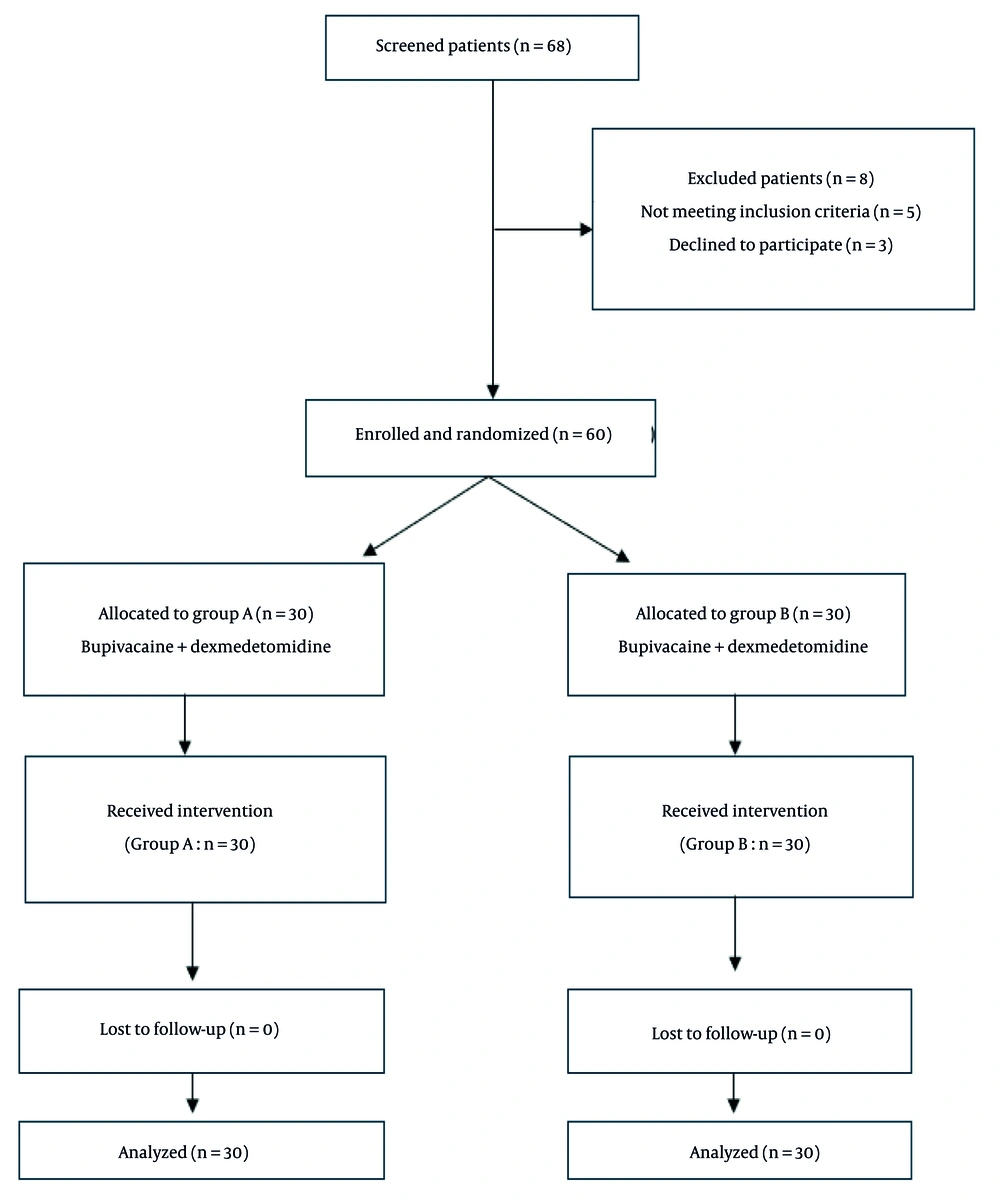
Data were collected, coded, tabulated, and then analyzed using the SPSS software package (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp., 2013). Numerical variables were presented as mean (standard deviation), while the NRS (as an ordinal variable) was presented as median (Q1 - Q3), and categorical variables were presented as frequency (%). Comparisons of numerical variables were conducted using the unpaired t-test or Mann-Whitney test as appropriate, with the latter also used for the analysis of NRS. Fisher’s exact test or chi-square test were used for comparisons of nominal variables. Kaplan-Meier survival analysis with a log-rank test was used to compare the time to first rescue analgesia. Any difference with a P-value < 0.05 was considered statistically significant (Figure 1).
Comparison between the two groups according to sociodemographic data showed that baseline sociodemographic and clinical indices demonstrated an absence of statistically discernible variance (Table 1).
| Baseline Characteristics | Group A (N = 30) | Group B (N = 30) | P-Value |
|---|---|---|---|
| Sex | 1.000 | ||
| Female | 16 (53.3) | 17 (56.7) | |
| Male | 14 (46.7) | 13 (43.3) | |
| ASA | 0.382 | ||
| 1 | 6 (20.0) | 10 (33.3) | |
| 2 | 24 (80.0) | 20 (66.7) | |
| Age | 59.83 ± 8.192 | 59.20 ± 6.509 | 0.741 |
| BMI | 26.27 ± 3.248 | 26.53 ± 2.596 | 0.727 |
| Duration of surgery (min) | 119.20 ± 15.334 | 116.03 ± 16.699 | 0.447 |
Abbreviation: ASA, American Society of Anesthesiologists.
a Values are expressed as No. (%) or mean ± SD.
Postoperative pain perception (NRS) among the studied groups showed an increase in NRS in both groups within a period of 24 hours, but a statistically significant increase was observed in group B compared to group A according to NRS after 30 minutes in the PACU (median 0 vs. 0, P = 0.002), after 4 hours (2 vs. 1, P = 0.011), after 8 hours (4 vs. 3, P = 0.006), and after 24 hours (5 vs. 3, P < 0.001) at the ward. No significant differences were found between the groups at 12, 16, and 20 hours (Table 2).
| NRS | Group A | Group B | P-Value |
|---|---|---|---|
| 0 (30 min in PACU) | 0 (0 - 0) | 0 (0 - 1) | 0.002 |
| 1 (after 4 h at ward) | 1 (1 - 2) | 2 (1 - 3) | 0.011 |
| 2 (after 8 h at ward) | 3 (2 - 3) | 4 (2 - 4) | 0.006 |
| 3 (after 12 h at ward) | 4 (3 - 4) | 3 (3 - 4) | 0.419 |
| 4 (after 16 h at ward) | 3 (3 - 4) | 4 (3 - 4) | 0.127 |
| 5 (after 20 h at ward) | 3 (3 - 4) | 3 (3 - 4) | 0.063 |
| 6 (after 24 h at ward) | 3 (3 - 5) | 5 (4 - 5) | < 0.001 |
Abbreviation: NRS, Numerical Rating Scale.
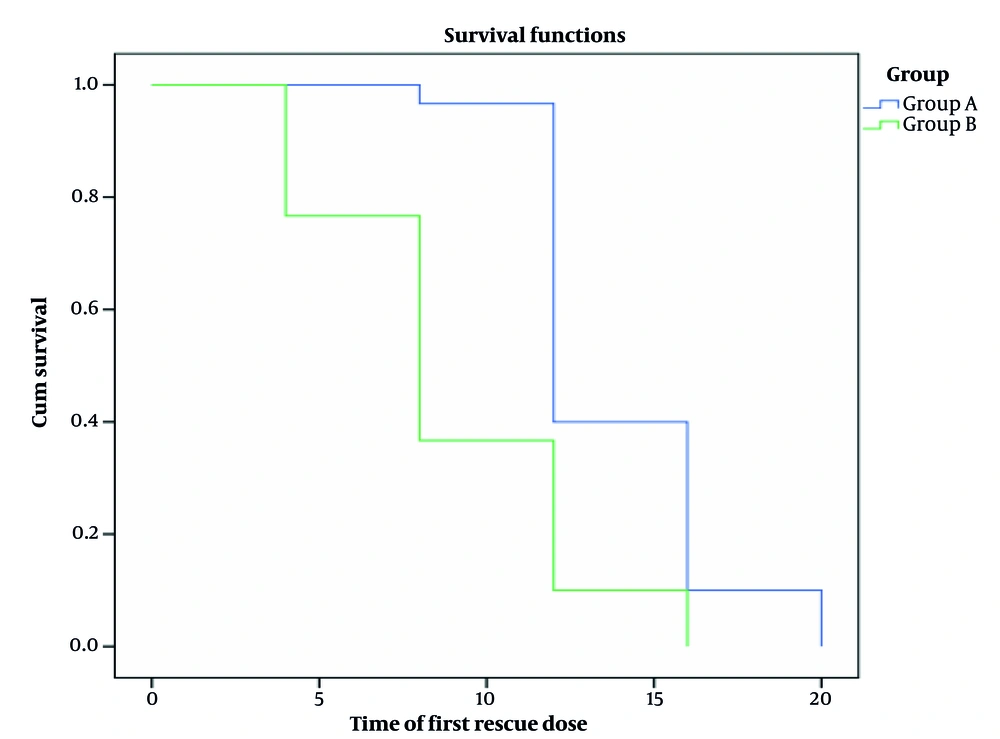
Total narcotic dose in milligrams and time to first rescue analgesia in the studied groups showed a significant increase in group A compared to group B regarding the time to first rescue analgesia (13.87 ± 2.92 hours vs. 8.93 ± 3.74 hours, P < 0.001). Additionally, there was a significant increase in total opioid consumption in group B (16.00 ± 5.63 mg) compared to group A (8.17 ± 2.78 mg) during the first 24 hours (Table 3).
| Groups | N | Values (Mean ± SD) | P-Value |
|---|---|---|---|
| Time of first rescue dose of Nalbuphine (per h) | < 0.001 | ||
| Group A | 30 | 13.87 ± 2.921 | |
| Group B | 30 | 8.93 ± 3.741 | |
| Total Opioids consumption in the first 24 h in mg | < 0.001 | ||
| Group A | 30 | 8.17 ± 2.780 | |
| Group B | 30 | 16.00± 5.632 |
Kaplan-Meier analysis showed a significant increase in group A compared to group B regarding the time to first rescue analgesia (Figure 2).
Comparison between the two groups according to complications showed that the incidence of hypotension was higher in group B (76.7%) compared to group A (50.0%), with a P-value of 0.060. Bradycardia occurred in 20.0% of patients in group A and 3.3% in group B (P = 0.103). Postoperative nausea and vomiting (PONV) were reported in 36.7% of group A and 33.3% of group B (P = 1.000). None of these differences were statistically significant (Table 4).
| Complications | Group A (N = 30) | Group B (N = 30) | P-Value |
|---|---|---|---|
| Hypotension | 0.060 | ||
| No | 15 (50.0) | 7 (23.3) | |
| Yes | 15 (50.0) | 23 (76.7) | |
| Bradycardia | 0.103 | ||
| No | 24 (80.0) | 29 (96.7) | |
| Yes | 6 (20.0) | 1 (3.3) | |
| PONV | 1.000 | ||
| No | 19 (63.3) | 20 (66.7) | |
| Yes | 11 (36.7) | 10 (33.3) |
Abbreviation: PONV, postoperative nausea and vomiting.
a Values are expressed as No. (%).
In conclusion, the combination of bupivacaine with dexmedetomidine provided superior analgesia compared to bupivacaine with dexamethasone, as reflected by significantly lower NRS pain scores (30 minutes in PACU, 4, 8, and 24 hours postoperatively), longer duration before the first analgesic requirement, and reduced total opioid consumption, which supports its clinical benefit in early postoperative pain control.
5. Discussion
The PENG block has demonstrated promising results as a regional technique for enhancing analgesic efficacy in patients undergoing hip operations. It functions by anesthetizing the sensory branches of the femoral, obturator, and accessory obturator nerves, all of which contribute to the innervation of the anterior hip capsule (2). Dexmedetomidine improves cognitive performance in addition to its sedative, analgesic, and anxiolytic effects through the α2-AR (8).
The current study was undertaken to conduct a comparative appraisal of the analgesic profiles conferred by bupivacaine when co-administered with either dexmedetomidine or dexamethasone as adjuvants within the framework of PENG blockade for postoperative nociceptive modulation in hip surgery. The findings revealed that the cohort receiving the bupivacaine-dexmedetomidine combination (group A) exhibited a statistically robust attenuation of postoperative pain scores as measured by the NRS, in comparison to the bupivacaine-dexamethasone group (group B). Furthermore, group A demonstrated a pronounced prolongation in the latency to first rescue analgesia, alongside a significant reduction in cumulative analgesic consumption within the first 24-hour postoperative window, thereby indicating a superior and sustained analgesic profile.
The superior performance of dexmedetomidine in this study may be explained by its effect as an α2-adrenergic receptor agonist, which reduces nerve excitability and prolongs local anesthetic effects. In contrast, dexamethasone acts primarily through anti-inflammatory pathways, which may be less influential in the specific anatomical distribution and neurophysiology of the PENG block. While the differences in NRS scores between the two groups were statistically significant at several time points, the actual numerical differences were modest (mostly 1 - 2 points). Therefore, although dexmedetomidine demonstrated superior analgesia in a statistical sense, the clinical relevance of these differences may vary depending on individual patient perception. For elderly patients, even minor reductions in pain can improve overall comfort, reduce the need for opioids, and facilitate early mobilization.
While the differences in postoperative pain scores and time to first analgesia between the dexmedetomidine and dexamethasone groups were statistically significant, they also appear to be clinically relevant. The prolonged analgesia observed with dexmedetomidine, along with reduced opioid consumption, may contribute to improved patient comfort, decreased opioid-related side effects, and enhanced recovery in the early postoperative period. These effects can offer meaningful benefits in clinical practice, particularly in vulnerable elderly populations undergoing hip surgery.
The findings of this study align with those of Nagaraju et al. (9), who examined the comparative efficacy of dexmedetomidine (group D) and dexamethasone (group X) as adjunct pharmacologic agents in enhancing the analgesic profile of ultrasound-guided supraclavicular brachial plexus blockade in patients undergoing upper limb surgeries. The results showed that the time needed for the first analgesic requirement was significantly higher in group D than in group X (935.38 ± 129.01 vs. 810.66 ± 107.01 minutes, P < 0.001). The mean VAS score was significantly lower in group D compared to group X in the first 24 hours (2.98 ± 0.80 vs. 3.427 ± 0.7409, P = 0.005), and the total tramadol requirement in the first 24 hours in group D was significantly lower than in group X (161.60 ± 51.085 vs. 197.60 ± 50.611 mg, P = 0.001). Thus, our findings support these results even though the block type and surgical context differed.
Gao et al. (10) explored the differential analgesic impact of incorporating dexmedetomidine or dexamethasone as adjunctive agents to ropivacaine in ultrasound-facilitated erector spinae plane blockade for patients undergoing pulmonary lobectomy via endoscopic thoracic visualization techniques during the first 72 hours postoperatively. The subgroup receiving dexmedetomidine co-administration demonstrated less pain intensity at all-time points. Furthermore, the temporal threshold to initial patient-controlled analgesia activation was markedly extended in the dexmedetomidine group (mean ~27 hours) relative to the dexamethasone group (~20 hours) and the ropivacaine-only group (~14.5 hours). Our findings support these results even though the block type and surgical context differed, and this study had a longer postoperative follow-up period (72 hours) than ours (24 hours).
Moreover, Khaleeq et al. (11) showed that dexmedetomidine is more effective than dexamethasone as an adjuvant to local anesthetics in ultrasound-assisted supraclavicular brachial plexus blockade for upper limb procedures. This was evidenced by the significantly increased time to request analgesia post-operatively in the dexmedetomidine group compared to the dexamethasone group (1015.5 ± 245.98 vs. 807.5 ± 196.74 minutes, P = 0.001), which was consistent with this study's results.
However, Coviello et al. (12) conducted a comparative analysis involving the incorporation of either dexamethasone or dexmedetomidine as adjuvants to a dual-agent anesthetic base consisting of lidocaine and ropivacaine in the setting of ultrasound-guided popliteal sciatic nerve block for hallux valgus correction. The study reported no significant difference in time to first rescue analgesia between the dexmedetomidine and dexamethasone groups within 48 hours. This discrepancy may be explained by methodological differences, including the use of a dual-agent local anesthetic mixture (lidocaine and ropivacaine), the type of block, and possible confounding pharmacodynamic interactions, which may have reduced the ability to detect differences between the adjuvants. Additionally, this study had a longer postoperative follow-up period (48 hours).
In contradistinction to that, Aliste et al. (13) found that dexamethasone provided a longer analgesic duration than dexmedetomidine when used with lidocaine, bupivacaine, and epinephrine in infraclavicular blocks (22.2 ± 3.6 vs. 16.9 ± 3.9 hours; P < 0.001). This observed difference is likely attributable to the inclusion of lidocaine and epinephrine in the administered mixture. Iyengar et al. (14) investigated the effectiveness of dexmedetomidine compared to dexamethasone as supplementary agents to bupivacaine in infraclavicular blocks for patients undergoing upper limb surgeries. The findings indicated that the addition of dexamethasone as an adjuvant to 25 mL of 0.5% bupivacaine provided enhanced postoperative analgesia and a longer duration until the time to first rescue analgesia compared to dexmedetomidine (990 ± 170.23 minutes versus 518 ± 150.12 minutes; P ≤ 0.0001). This discrepancy might be attributable to the different block site, as well as their use of 0.5% bupivacaine in 25 mL, while our study used 20 mL of 0.25% bupivacaine.
Our study has some limitations. First, although the sample size was calculated appropriately, a larger sample size may yield better results. Second, all patients received spinal anesthesia before the block, which could have influenced early postoperative pain scores. Third, the study did not include long-term follow-up beyond 24 hours to assess delayed analgesic differences. Finally, no sensory testing was done to confirm the exact dermatomal spread of the PENG block.