1. Context
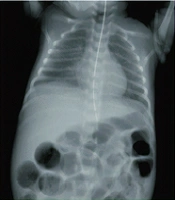
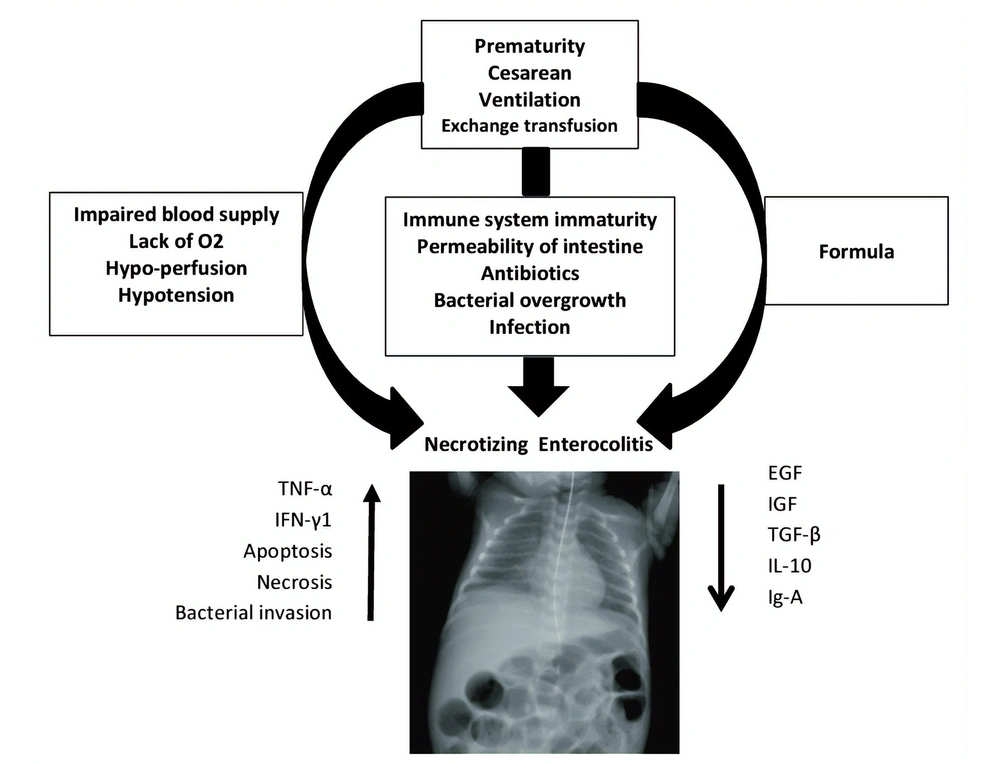
Necrotizing enterocolitis (NEC), as a devastating and life-threatening gastrointestinal tract disease, mostly affects infants born with low (or very low) birth weight (1). About 5 to 10% of premature infants with less than 1500 gr of born weight develop NEC (2). Alongside prematurity as the most significant risk factor, black ethnicity, assisted ventilation, cesarean section, surfactant therapy, exchange transfusion, hypotension, and gestational diabetes may contribute to invasion of pathogens, deep injuries, and demolitions of the bowel wall in NEC (3). In addition, prognostic factors including intestinal bacterial overgrowth, sepsis, impaired blood supply, lack of oxygen supply, intestinal damages, inappropriate nutrition (e.g., fortified supplements) contribute to infants' vulnerability to NEC. Histopathologically, a compromised immune system keeps the gastrointestinal tract of infants incomplete and is recognized as a trigger. A brief overview of NEC is provided in Figure 1 (4, 5).
The mortality rate of NEC is notably high. Besides, the mortality rate of those who need surgical interventions is up to 50% (6). Infants who survive NEC have various short- and long-term complications such as intestinal stricture, short bowel syndrome, cholestasis, liver disease, and feeding difficulties (6). In addition, they suffer from some degrees of poor growth and increased risk of neuro-development impairments (7). While in the early 2000s, NEC was the most common gastrointestinal emergency in neonatal intensive care units (NICU) in the United States (8), fortunately, its incidence has declined in recent years. This decline can be attributed to paying special attention to purposeful preventive efforts such as encouraging all mothers to feed their baby by mother’s own milk (MOM) or preparing the donor human milk (DHM) as an alternative option (9-12). Multiple studies mentioned the prospective advantages of mother’s milk, particularly in the first months of the newborn’s life. Bio-components of human milk support the immune system of newborns. Nevertheless, many mothers of preterm infants’ have difficulties in breastfeeding their infants because of their breast immaturity due to preterm labor. Some of them also suffer from problems in breastfeeding initiation. In spite of these problems, several countries still do not have human milk banks to provide DHM. Therefore, sometimes commercial and fortified milk are preferred options (13). Human milk bio-components are remarkably different from fortified supplements and formulas in terms of immune factors, minerals, vitamins, and cellular contents, with the consideration that these are actually much more abundant in human breast milk (14). Moreover, the donor milk pasteurizing process, including freezing or high-temperature exposure, inevitably causes significant differences, particularly in its immunity power of DHM, compared to the MOM. The current study intended to review human milk bio-components' role in preventing NEC evaluated in the recent studies, and then make our discussion by taking a look at the clinical studies on the role of human milk in NEC.
2. Evidence Acquisition
2.1. Search Strategy
We systematically searched CINAHL, Embase, PubMed, and Cochrane databases in order to find relevant articles published from 1 January 2000 to 30 June 2016 using various combinations of the following search terms: "Breast Milk and Necrotizing Enterocolitis," "Donor Milk and Necrotizing Enterocolitis," and "Human Milk and Necrotizing Enterocolitis." To ensure comprehensiveness of the search, references of identified articles were also hand-searched. Due to the new advances in neonatology, such as a narrower diagnosis of NEC, early initiation and completion of full-feedings, as well as improved preterm formulas and HM fortifiers, the research team decided to limit the search to articles published after January 2000.
2.2. Inclusion and Exclusion Criteria
Articles were deemed suitable for review if 1- they involved premature infants receiving HM feedings, 2- a primary or secondary result was NEC, and 3- an original data-driven study was identified. The following studies were excluded: animal experiments, quality management programs, case notes, case series, recommendations, book chapters, and review papers. According to the abovementioned criteria, of 393 identified and evaluated articles, 29 were excluded.
For definite exclusion criteria and duplication, abstracts of papers found by the initial search were screened. The full-text of the remaining publications was then obtained and fully reviewed, and evaluated for their eligibility. In case of a disagreement, a consensus was reached through discussion. Each reviewer used a standard table for all final articles to collect data, including demographic detail, research design, HM type, dosage given, result, and level of evidence.
2.3. Quality Assessment
Using the Standard of Evidence grading scale from the Centre of Evidence-Based Medicine, Oxford, United Kingdom, the degree of evidence was calculated. This approach assesses the quality of evidence on the basis of a numerical scale (1-5) and an alphabetical scale (a-c). Instead of diagnosis or prognosis, the standard of evidence for this analysis focused on therapy or prevention. The standard of proof for each analysis was separately measured and graded by each reviewer, and findings were compared and debated before consensus was achieved.
2.4. Data Synthesis and Reporting
For each sample, the form and dosage of HM were recorded. The experiments were divided into 3 groups to promote data synthesis: studies that have investigated a DHM, an EHM diet, and the HM (MOM and/or DHM) dosage.
3. Results
Breast milk, in addition to providing an energy source for newborns, plays a major role in supporting the infant's immune system. The most crucial bio-components found in human milk are listed in Table 1. In this section, we have reviewed the most critical and well-recognized constituent elements of human milk, which have key effects on protecting infants against NEC (Table 1).
| Bio-components | Futures | Function | References |
|---|---|---|---|
| Cells | |||
| Macrophages | Contain of soluble Ig-A | Improve B and T cell function | (15, 16) |
| Neutrophils | Decreased polarity and motility; High expressed levels of CD11b and L-selectin | Mild role in development of infant’s immunity | (17, 18) |
| Lymphocyte T | Over 80 percent of total CD4 and CD8 types | Maturation of infant’s T cells; Thymus development; Protection against viruses | (19-21) |
| Lymphocyte B | Class-switched memory B cell phenotype mostly CD44+ and CD38+ | Spontaneously Ig secretion; Development of infants humeral immune system | (22) |
| Stem cells | - | Regeneration and repairmen | (23) |
| Growth factors | |||
| IGF | - | Stimulation of development; Increased hemoglobin (RBC); Immune system modulation | (24-27) |
| NGF | - | Stimulation of neural development and maturation | (24-27) |
| EGF | - | Stimulation of cell layers proliferation and maturation | (24-27) |
| Prebiotics | |||
| Hmos | Indigestible carbohydrates | Increase nutrient availability of intestinal flora; Bacterial anti –adhesive reduced intestinal permeability; Prebiotic property | (28, 29) |
| Bifidobacterium | Gram- positive, none- motile bacteria | Providing infant’s normal gut flora | (30) |
| Lactobacillus | Gram-positive, facultative anaerobic or microaerophilic | Providing infant’s normal gut flora | (30) |
| Proteins | |||
| Lysozyme | - | Bactericidal | (31) |
| Lactoferrin | - | Maturation of Intestinal Cells; Immune System Development; Iron absorption bactericidal | (32, 33) |
| Amino acids | |||
| Cytroline | Nitric oxide cycle regulator | Increased Intestinal blood supply; Free radical inhibition | (34, 35) |
| Arginine | Nitric oxide cycle regulator | Increased intestinal blood supply; Free radical inhibition | (34, 35) |
| Immunoglobulins | |||
| Ig-A | Soluble Ig-A (sig-A) | Pathogen anti-adhesive | (36-38) |
| Ig-G | Igg1, igg2, igg3, igg4 | Phagocyte cells activation; Anti-inflammation; Activate allergen response cycle | (36, 38) |
| Ig-M | - | Agglutination, | (36, 38) |
| Cytokines | |||
| IFN-Y | - | Stimulation of Th1 cells; Pro-inflammatory characteristic | (25, 27) |
| TGF-β2 | - | T cell phenotype switch promotion | (27, 39, 40) |
| TNF-α | - | Activation of inflammatory immune factors | (27, 41, 42) |
| Interleukins | |||
| IL-1β | - | Lymphocyte activating factor | (27, 43) |
| IL-6 | - | Stimulation of B cell types pro-inflammatory | (40, 44) |
| IL-7 | - | Development of thymus | (45) |
| IL-8 | - | Neutrophils recruitment | (44, 46, 47) |
| IL-10 | - | Th1 cells repression; Stimulation of Ab production | (44, 48) |
3.1. Lactoferrin
Lactoferrin, known as a plentiful multifunctional glycoprotein in human milk, plays a key role in the innate immune system, with the features of being antibacterial, antiviral, anti-parasitic, and anti-allergic (13, 49, 50). By disrupting the membrane of bacteria, Lactoferrin inhibits bacterial infections (49, 50). It also plays an important role in the improvement of cell layers of the intestine (51), proliferation and differentiation of B and T lymphocytic cells (52), and absorption of ferric iron (15). Some randomized controlled trials demonstrated that the oral Lactoferrin, as a prophylactic treatment in infants, could lessen the risk of NEC (16, 17). A randomized placebo-controlled trial study with 2203 participants performed in 2019 reported that milk fortified by bovine lactoferrin was associated with reduced risk of mortality as well as delayed onset of sepsis in preterm infants (18).
3.2. Lysozyme
Lysozyme, as a strong bio-active enzyme in human milk, is known for its antibacterial and anti-viral properties (19). Lysozyme binds to polysaccharides of bacterial cell layers and cleaves them (20). Paneth cells are the human gastrointestinal secretory epithelial cells that produce various antibacterial peptides, including lysozyme (21). Some case-control studies reported decreased levels of Paneth cells in the intestinal cell layers of newborns suffering from NEC compared to healthy newborns (22, 23).
3.3. Immunoglobulins
The most abundant immunoglobulins in human milk are IgA, IgM, and IgG. (24). IgM and IgG are less prevalent, and their immunity enhancement is lower than IgA (24, 25). IgA is the most abundant human milk antibody. Besides, it is a noticeable content of the total protein of colostrum. Breast milk IgA has an important antimicrobial defense mechanism in the neonatal gastrointestinal tract by inhibiting pathogens, especially Enterobacteriaceae, from binding to the mucosal surfaces or neutralizing microbial toxins (25, 26). As IgA is not available in formula and fortified milk, their frequent consumption causes significant insufficient immunity. Although some studies showed that in NEC patients, there is an elevated level of IgA-unbound Enterobacteriaceae (27). Foster j. and Cole M. did not show any effect on the prevention of NEC (28).
3.4. Epidermal Growth Factor
Maternal milk and colostrum contain epidermal growth factor (EGF), which inhibits maturation of the intestinal mucosal barrier (5, 29). A clinical study has illustrated the significance of EGF in protecting the intestine against NEC (30). Intestinal epithelial homeostasis is achieved by the main role of EGF in repairing and developing it, which is an important barrier against pathogens (29, 31). On the other hand, significantly low serum levels of EGF in infants with NEC indicates the importance of EGF as a reliable marker that contributes to NEC (32).
3.5. Transforming Growth Factor-β2
High levels of transforming growth factor-β2 (TGF-β2) in breast milk is known as a stromal cell-derived factor, affecting intestinal maturation and immune response (33). TGF-β2 regulates T-cells responses as an invigoration of cell-mediated immunity system, which plays an important role against NEC (33, 34). Breastfeed infants have higher serum levels of TGF-β2 than formula-feeding infants (35). A case-control study, using investigatory analyses, concluded that NEC patients did not have TGF-β (36).
3.6. Antioxidants
Hypo-perfusion, lack of oxygen, ischemia, sepsis or microbial infections, and inflammation are associated with increased levels of reactive oxygen species, hence turning cells into the oxidative stress state and consequently producing free radicals as a cell interrupter (37). Vitamin E, Carotene, Citrulline, Glutamine, Arginine, and Melatonin are effective radical scavengers and antioxidant agents exerting pleiotropic actions (38-41). About 4% of amino acids in human milk are free amino acids (42). Citrulline and Arginine are essential for the formation of nitric oxide (NO), which regulates intestinal blood flow by accelerating the availability of antioxidant and anti-inflammatory factors. Therefore, it is argued that the reduced level of circulatory amino acids, such as Glutamine and Arginine, is associated with an elevated risk of NEC development (43). A systematic review study by Mitchell K and Lytle A. suggested that L-arginine supplementation is an appropriate intervention to prevent NEC (41).
3.7. Human Milk Oligosaccharides
Human milk oligosaccharides (HMOs) are the most plentiful dissolved and emulsified components, after lactose and lipids, found in the breast milk, which are known as multifunctional and indigestible carbohydrates (44-46). HMOs prevent NEC by supporting epithelial cells, inhibiting bacterial adhesion, and speeding up the healing process of the intestine by precipitating the turnover of crypt cells. HMOs also have a modifying role concerning bacterial infections of the intestines (47). Several studies reported the undeniable role of HMOs against NEC. So far, the exact molecular mechanisms of the influence of HMOs are not completely revealed (48, 53).
3.8. Bifidogenic Factors
Human milk contains sufficient prebiotics for infants' intestinal normal flora, including Bifidobacterium species (known as bifidogenic factors) (54, 55). Bifidogenic effect of human milk not only depends on various components such as lactoferrin, lactose, nucleotides, and HMOs, but also relies on the regulated concentration of phosphate, pH, and proteins (56). HMOs are the main bioactive component with prebiotic properties (57). These factors regulate intestinal epithelial homeostasis, inhibit over-colonization of invasive groups of bacteria, and decrease the risk of NEC (58).
3.9. Probiotics
Prebiotic characteristics of human milk affect the type and species of the microbial in the infant’s body. Probiotics protect the gut by inhibiting over colonization of pathogenic bacterial groups (59). Gut flora changes are due to the administrations of Antibiotics; in fact, it increases the risk of NEC (60, 61). The quantity of Bifidobacterium, as the main intestinal normal flora, is less abundant in premature infants (62); In a case-control study, infants suffering from NEC had fewer Bifidobacterium (in their intestine) compared to the controlled group (63). It is well proved that breastfeeding infants have a greater abundance of Bifidobacterium and Lactobacilli in their stool culture. In contrast, formula-fed infants had a higher number of Enterobacteriaceae and Bactericides in their stool culture (64). Likewise, the overgrowth of Enterobacteriaceae is correlated with NEC (65).
4. Discussion
The amazing bio-components of human milk can inhibit NEC development in infants, whether they are coming from MOM or DHM, in contrast to Formulas, which are not rich in any of these curtail factors. Nevertheless, there are considerable differences between MOM and DHM.
The milk collected from donors undergoes different processes, like exposure to light, air, different temperatures, multiple cycles of freezing, and pasteurizing in order to ensure the safety of the product. Not only do these procedures alter the bio-components of MOM, but also they may become inactivated or useless over time. We should bear in mind that DHM is received from mothers with several months of breast milk expression, which is actually different in ingredients compared to the first-days expression milk. (66-69). The best example is the changing level of the human milk lactoferrin during lactating in both term and preterm mothers (70). It has been demonstrated that in the mothers' first days of lactating, Lactoferrin is at its highest levels (9 gr/L), then it drops gradually during the first month to half and subsequently to 2.5 gr/L by the end of the second months (50). By the way, premature infants may need to use DHM due to the inefficacy of their mother’s milk to meet their baby’s needs. Additionally, a study on extra premature infants indicated that merely 30% of mothers could meet their infants' needs during the hospitalization in NICU (71, 72). Nonetheless, it seems that DHM might be used as a suitable temporary substitute for preterm infants, whose mothers have not yet achieved sufficient breastfeeding (73).
The use of the formula is expected in mothers who cannot provide a sufficient amount of milk, living in non-DHM banks counties. A prospective study on 926 preterm infants reported that feeding options for babies were either only human milk, human milk plus formula, and only fed formula. This interventional change in nutrition was introduced in early post-birth. By evaluating the 31 confirmed NEC cases, they found that confirmed NEC has up to ten-fold probability in babies fed with the only formula, as compared to those receiving exclusively human milk. Also, it is reported that confirmed NEC has a three-fold commonality in those who received formula plus human milk. Additionally, in infants born > 30 weeks of gestational age, feeding with the formula (without human milk) increases the risk of NEC by 20 times compared to feeding with human milk (MOM and/or DHM) (74, 75). In the same vein, a clinical trial study demonstrated that preterm infants with less than thirty weeks of gestational age were more affected by NEC when nourished by DHM and formula in comparison with solitary MOM fed infants (76). In addition, two studies vividly showed that infants with a nutrition history of human milk manifested much milder signs and symptoms of NEC (77, 78). In other words, it can be argued that breast milk not only has an important protective role but also alleviates the severity of NEC symptoms, whether is used as a supplement to formula feeding (79).
On the other hand, the feeding introduction timing and volume to newborn, has an important effect on outcomes or risk of NEC. The early introduction of breastfeeding is related to abate in the incidence of NEC. A retrospective assessment of 1272 infants showed that the probability of NEC or death after two weeks was declined by an extent of 0.83 for each 10% rise in the quota of total intake of human milk (80). Besides, in a research on 1028 premature infants, focusing on the comparison between those who developed NEC and circumstantially matched controls, it is shown that if the infants had received MOM for less than a week, there was a four-time elevated risk for NEC. This indicates that even a few days of being fed by MOM can have a protective effect against NEC (81). Also, analyzed data suggest that, in an extremely premature population, the protective features of the early onset of human milk last for a long time (73).
It worth noting that the regulation of the consumption volume of milk is one of the important keys in infants' health. A study has shown that a 100 ml/kg increase in the consumption of human milk, was associated with decreased risk of NEC. This decline was specifically observed in infants born with less than 1000 gr weight, and only if the human milk consumption had taken place in the first two weeks of life (82). Accordingly, another research has come to this conclusion that maternal feeding containing at least 50% human milk in the first two weeks of life is associated with decreased incidence of NEC (by about six-folds) (83). Also, the importance of dose-dependent preserving effects of human milk has been widely shown elsewhere. By comparing those fed on a comprised diet with less than 50% MOM, those receiving a diet with more than 50% MOM in the first two weeks after birth rendered an 83% decrease in the subsequent progression of NEC in infants. Astonishingly, initiating MOM even at a dosage of around 45% in the first 5 days can decrease the incidence of later NEC, sepsis, and/or death within the first two-month of life (84). In addition, a systematic review study on 3753 infants born with very low weight has assessed slow and faster rates of feeding in a comparison manner. It is concluded that infants fed more quickly had a lower risk of severe infections (57). While the daily dose is required to detect the difference in health benefits, this amount should not be the ultimate goal (13); as shown by Berseth and colleagues, increasing the feeding volume, instead of maintaining a prolonged feeding volume, in the first 10 days of life is associated with increased risk of NEC, and this isn't followed by improvement in motor function nor feeding tolerance (85).
4.1. Conclusions
Human milk, as a food choice of the newborn, consists of an incredible variety of biological components that reduce the risk or severity of Necrotizing Enterocolitis (NEC). Based on these facts, recent evidence distinctly emphasized the preference of breast milk to any alternative food types. Even though DHM is human milk but it is not the same in terms of quantity and quality of MOM’s ingredients that play an important role in supporting the innate immune system as well as preventing NEC development. Despite the benefits of MOM, there are some infants that cannot benefit from breastfeeding. DHM or MOM may be appropriate options for these infants, even in a small amount, compared to only formula feeding. Moreover, the onset of early feeding with human milk in a suitable portion and volume can improve the outcomes. In conclusion, the most preferable food for newborns is MOM that yields the best results.