1. Background
The International Children’s Continence Society accepted bladder-bowel dysfunction (BBD) as a descriptive term for a combined disorder of bladder and bowel in a child. BBD comprises lower urinary tract symptoms (LUTS) and bowel dysfunction. BBD includes urological dysfunctions such as overactive bladder (OAB), underactive bladder, and dysfunctional voiding (1). The prevalence of OAB has been cited in studies, ranging from 8 - 12% in 5 - 10-year-old children (2) to 16.5% in 5 - 13-year-old children (3). Dry OAB is more common in older children, whereas wet OAB is more prevalent in younger ones (4). OAB is one of the common causes of lower urinary tract dysfunction (LUTD) in children. Children with OAB complain of daytime frequency, the urgency with or without urge incontinency, nocturnal enuresis, and urinary tract infection (1, 2, 5). OAB symptoms may continue to adulthood (6). Bowel dysfunction represents primarily constipation and/or encopresis. Constipation affects bladder function by mechanical compression on the bladder neck and results in post-void residual volumes. Rectal fecal mass also aggravates urinary symptoms like urge incontinence and frequency (7). Recent studies have revealed that BBD is an important risk factor in many children with recurrent urinary tract infection (UTI) and renal scarring (8).
In recent years, obesity has been a significant health problem with many co-morbid disorders. The prevalence of overweight and obesity has dramatically increased in children (9). Obese boys may suffer from poor body image, micro-penis, and decreased quality of life (10, 11).
A study compared the prevalence of OAB symptoms on obese people with healthy controls. Frequency in obese cases and feeling of incomplete bladder emptying were more reported in obese women (12). In overweight and obese women, intra-abdominal pressure increases, leading to weakness of the fascia and pelvic floor muscles and causing urgent and stress urinary incontinence (13). In healthy community children, the prevalence of overweight and obesity is reported 14.0% and 10.0%, respectively. The urgency is prevalent in obese children. Also, young age and obesity are predisposing factors for OAB. Gender, overweight, obstructive sleep apnea and stressful events do not affect bladder symptoms (14).
There is some ambiguity regarding the relationship between OAB and obesity, and we did not observe many obese OAB patients in the clinic. Thus, we conducted this study on our OAB patients and compared their BMI percentile with healthy controls. We also assessed the relationship between the bladder symptoms and BMI percentile.
2. Objectives
This study aimed to evaluate the relationship between overweight and obesity with OAB in children.
3. Methods
This cross-sectional study was conducted at the Qazvin Children Hospital, Iran, from January 2017 to January 2018. We compared 56 children aged 3 - 16 years with OAB symptoms with 56 healthy children regarding overweight and obesity. Patients aged 3 to 16 years who were admitted to the nephrology clinic of the Qazvin Pediatric Hospital for voiding disturbance were assessed by a pediatric nephrologist. Children in the case group were diagnosed with OAB if they met the following parameters: (1) Existence of urge incontinence or urgency, frequency, nocturia or nocturnal enuresis, with or without constipation, and (2) fulfilled OAB symptoms in the past three months. Children with neurocognitive disorders, isolated nocturnal enuresis, and anatomical abnormalities of the urinary tract system, and those with acute or chronic renal failure were excluded. All OAB patients visited within one year were entered the study consecutively. Healthy children were selected by group matching, sex, and age among those visited in the general pediatric. For patients with OAB and children with no history of voiding dysfunction and UTI, the OAB questionnaire was completed.
Sampling was being continued successively until the expected sample size (see the Supplementary File for the formula used to calculate the sample size) (14).
A three-day bladder diary was provided to the patients to record frequency, urgency, and urinary incontinence. Frequency was determined by more than eight urinations per day. The urgency was defined as a sensation of voiding due to involuntary detrusor contraction prior to micturition, which may lead to leakage and urge incontinence (15). Also, urge incontinence was defined as an involuntary leakage accompanied or preceded by urgency (13). OAB was defined if the child had urgency with or without urge incontinence. For assessing defecation characteristics, we asked parents and children about frequent defecation per week, forceful defecation, feeling of incomplete emptying of the intestine, and hardness of the stool. We used Rome-III criteria to diagnose functional constipation (1). Questions were about urinary frequency, urgency, daily incontinence episodes, nocturia, and bowel habits.
Trained staff measured the patients’ height and weight using a standardized portable stadiometer and an electronic scale. Height was measured with a stadiometer with an error of one millimeter and weight with a German seca scale with an accuracy of one hundred milligrams. BMI was calculated using Quetelet’s index by dividing body weight in kilograms by the square of height in meters.
According to charts of NCHS/WHO (National Center for Health Statistics/World Health Organization), children with BMI-for-age ≤ 5th percentile are underweight, between 5th-85th percentile have normal weight, between 85th - 95th percentile are overweight, and over ≥ 95th percentile are obese. The parents’ level of education was categorized as bachelor's degree, technical junior college, associate degree, and junior high school or high school.
The Ethics Committee of the Research Department at the Qazvin University of Medical Sciences approved the study. All the parents were provided information regarding the research method in simple language and were asked to sign the informed consent form.
The statistical analyses were performed using the SPSS software version 18.0 for windows (SPSS Inc., Chicago, IL). Also, P-values lower than 0.05 were considered statistically significant. The study results are presented in the form of statistical tables and numeric indicators. For the continuous variables, mean and standard deviation were performed to present the data. Categorical variables were expressed by frequencies and percentages. The correlation testing was used (two-tailed Pearson's correlation coefficient analyses, or r), and if one variable was significant (relationships), we used the Spearman test to find OR and HR. These variables were analyzed with chi-square test and t-test. Chi-square test (or Fisher’s exact tests for cells with < 5 data) was used to examine the association between the two groups (patients vs. control) and compare clinical symptoms and BMI in the OAB group. For comparison of the continuous variables with normal distribution between the two groups, an independent t-test was used. The Mann-Whitney test was used to compare the means or medians of the two independent groups with non-normal distributions.
4. Results
In the present study, 112 children were investigated, 56 children with OAB symptoms and 56 healthy children. The case group comprised 38 girls (67.8%) and 18 boys (32.2%), and the control group included 45 girls (80%) and 11 boys (20%). The mean age was 7.71 ± 2.65 in the case group and 7.42 ± 2.40 in the control group (P = 0.55). There were no significant differences between the OAB patients and the healthy ones in the BMI percentile (Table 1) (P = 0.23). In both groups, there was no difference between girls and boys in terms of obesity and overweight (P = 0.99).
| BMI (≤ 5%) Underweight | BMI (6 - 85 %) Normal Weight | BMI (86 - 94%) Overweight | BMI (≥ 95%) Obese | P-Value b | |
|---|---|---|---|---|---|
| Case | 13 (23.2) | 35 (62.5) | 5 (8.9) | 3 (5.4) | 0.231 |
| Control | 11 (19.6) | 42 (75.5) | 3 (5.4) | 0 |
Comparison of Body Mass Index (BMI) Percentile in Case and Control Groups a
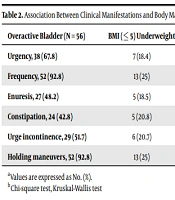
Among clinical symptoms, frequency, urgency, and holding maneuvers were the most frequent complaints. Frequency was more frequent in patients with normal weight compared to others (P < 0.001). Constipation was reported in 24 (42.8%) of the OAB children and was not associated with BMI. None of the patients had nocturia, although 27 (48.2%) of them complained of enuresis (Table 2). Seventeen (30.3%) children with nocturnal enuresis had night snoring. Among them, three were underweight, 12 had normal weight, and two were obese. Also, night snoring had no association with BMI percentile (P = 0.2). The prevalence of clinical symptoms did not differ in males and females. There was a history of UTI in 46 (82.1%) of the patients. Of note, 39 (84.8%) females in the OAB group were identified with a history of UTI, although not statistically significant. There were no significant differences in the level of parental education between the two groups (P > 0.05).
| Overactive Bladder (N = 56) | BMI (≤ 5) Underweight | BMI (6 - 85) Normal Weight | BMI (86 - 94) Overweight | BMI (≥ 95) Obese | P-Value b |
|---|---|---|---|---|---|
| Urgency, 38 (67.8) | 7 (18.4) | 25 (65.8) | 5 (13.2) | 1 (2.6) | 0.146 |
| Frequency, 52 (92.8) | 13 (25) | 33 (63.5) | 5 (9.6) | 1 (1.9) | 0.001 |
| Enuresis, 27 (48.2) | 5 (18.5) | 20 (74.1) | 1 (3.7) | 1 (3.7) | 0.324 |
| Constipation, 24 (42.8) | 5 (20.8) | 15 (62.5) | 2 (8.3) | 2 (8.3) | 0.846 |
| Urge incontinence, 29 (51.7) | 6 (20.7) | 17 (58.6) | 5 (8.8) | 3 (5.4) | 0.231 |
| Holding maneuvers, 52 (92.8) | 13 (25) | 33 (63.5) | 5 (9.6) | 1 (1.9) | 0.001 |
Association Between Clinical Manifestations and Body Mass Index (BMI) Percentile in Overactive Bladder (OAB) Children a
5. Discussion
LUTD affects 2 to 25% of the pediatric population (16), and most of them have OAB (17). The prevalence of OAB in children aged 5 to 14 years is about 9% (4). Previous studies have reported the association between obesity and OAB. Most of these studies evaluated the prevalence of OAB symptoms in obese children (4, 18-21), Other studies described that underweight or overweight children had higher scores for lower urinary tract problems, and the rate of obesity in children with lower urinary tract symptoms was reported to be 28.5% (22). We investigated overweight and obesity in children with OAB and found no difference between patients with OAB and healthy controls regarding overweight and obesity. The rate of obesity was low in the case and control groups. Studies about obesity prevalence in Iranian primary school students were reviewed, revealing that 7.18 percent (95% CI: 5.63-8.73) of students were obese, with obesity being more prevalent in boys than in girls (8.02% vs. 6.07%) (23). Most children in both groups had normal body mass index (BMI), and only three patients in the case group were obese compared to none in the control group. The rate of being underweight in the case and control groups was 23.2% and 19.6%, respectively, which is slightly higher than the average underweight prevalence (15.5%) in our country (24).
Our patients were not overweight or obese because of their racial differences and socioeconomic status compared to other societies. The population of our study was from the city and its surroundings, and a number of children suffered from some degree of malnutrition. Also, recurrent UTI in the OAB patients may interfere with somatic growth. On the other hand, the definition of obesity in some studies was different from ours. For example, Ardem defined mild obesity as obesity with BMI between 85% and 95% and severe obesity as obesity with BMI higher or equal to 95% percentile (19). Children with urinary incontinence refuse to drink fluids for fear of becoming wet. Inadequate fluid intake to prevent urinary incontinence can lead to UTI (25).
In our study, the prevalence of UTI in the OAB children was 82.1%, which was higher than that in other studies (26). Similar to other studies, UTI was more common in girls (18, 26-28).
Fraga and colleagues concluded that only symptoms related to bladder filling (the presence and severity of urinary incontinence and urgency) were associated with obesity (21). We found that frequency and holding maneuvers were more prevalent in normal BMI patients. However, more cases are needed to prove a definite association in this regard.
In our study, the prevalence of bladder symptoms did not differ between males and females. However, Chung reported that urge symptom was more common in girls (3), and in Batavia's study, urinary incontinence in girls and frequency in boys was more common (29).
Bladder and bowel disorders are closely related, and most commonly, constipation was higher in the OAB patients than in the general population (20, 30, 31). Children with constipation are 6.8 times more likely to have a lower urinary tract disorder (31). Constipation reduces the functional bladder volume and causes incomplete bladder emptying (32). On the other hand, treating OAB with anticholinergics can make constipation worse (33). In the present study, 42.8% of OAB cases were constipated. History of constipation was more common in obese children (11.8%) than in normal-weight children and adolescents (4.9%) (21). Veiga and colleagues reported that 54.9% of children in the hyperactive bladder and 29.7% of them in the control group complained of constipation, without any association between bladder symptoms and constipation (27).
We observed that the level of parental education did not differ in the OAB and healthy cases, but Yuksel reported less-educated parents of LUTD children (32).
The sample size in this study was too small to estimate the prevalence of obesity in the general population. However, it can be a preliminary study to further investigate weight distribution in children with OAB in our population. Given that 24/112 (21%) of the population are underweight, we are better set up to compare the rate of OAB in underweight children.
5.1. Conclusions
The present study showed no association between overweight and obesity with OAB. It seems that overweight and obesity play no role in this type of voiding dysfunction in children. The number of children is too small to make any meaningful comparison between children with normal weight and overweight/obese children.