1. Introduction
Interstitial lung disease (ILD) is a rare condition in neonates. Pulmonary interstitial glycogenosis (PIG) is categorized as an ILD (1).
PIG manifests its symptoms within a short period after birth (in a matter of weeks to months) (2). There is some evidence advocating the idea that PIG is a self-limited disease and usually detectable within six months of age (1). Histological evidence which contains glycogen-laden mesenchymal cells within the pulmonary interstitium is characteristic in PIG diagnosis (2). PIG expresses itself with diverse clinical symptoms such as tachypnea and hypoxia and may lead to acute respiratory failure in neonates (2). In this article, we presented a 1.5-year-old boy with radiologic and pathologic findings of PIG. During the 2-year follow-up, he had normal growth and development without supplementary oxygen requirement. In serial follow-ups of every 6 months, there were no cardiac problems, pulmonary hypertension, and other complications.
2. Case Presentation
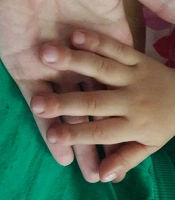
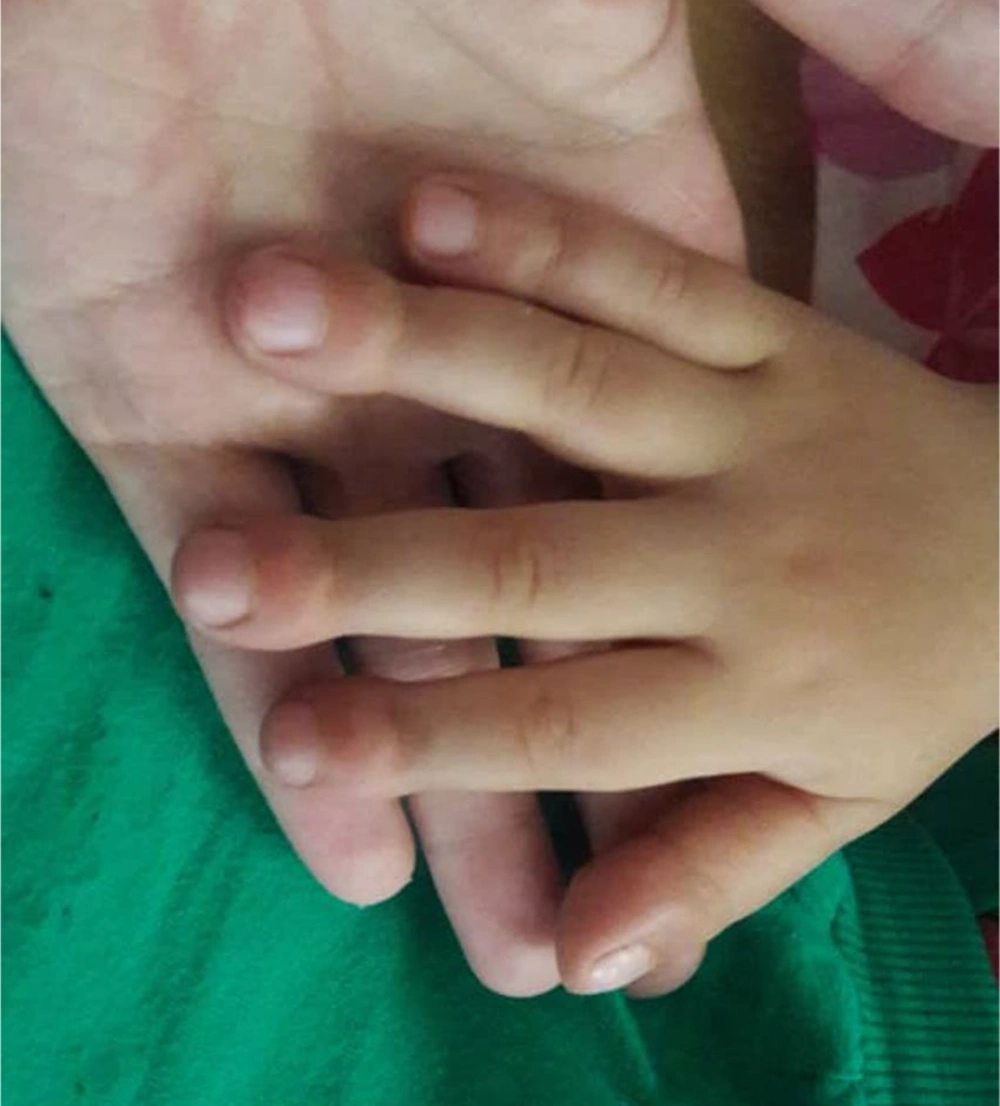
On 23rd August 2018, a 1.5-year-old boy who was generally in good health, referred to Taleghani hospital of Gorgan city with stage 4 clubbing in fingers and toes (Figure 1). There were no respiratory signs or extrapulmonary symptoms. There were no retractions on chest observation and no crackles on auscultation, but there was mild chest deformity in physical examinations. He was born by spontaneous vaginal delivery at 38 weeks of gestation after a normal pregnancy with 4200 g birth weight, and his growth and development were normal. He did not have pulmonary symptoms at birth. His neonatal period was not accompanied by respiratory distress.
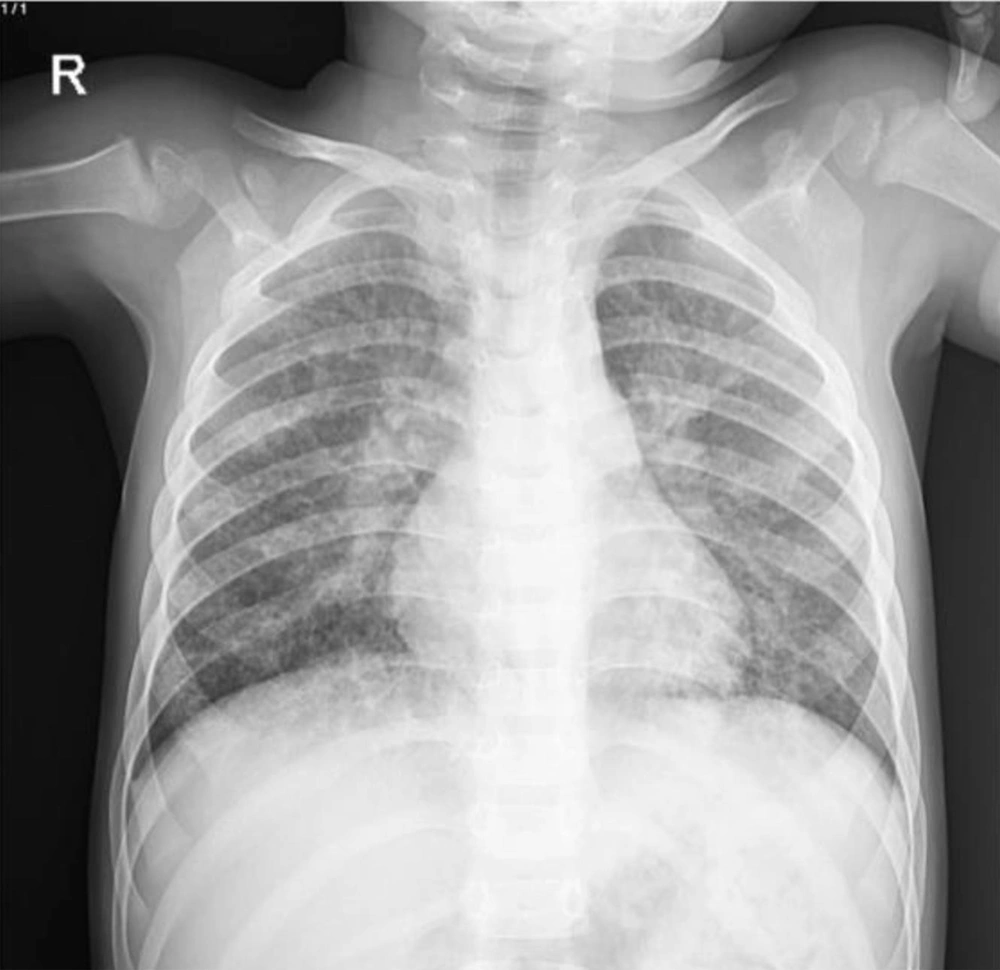
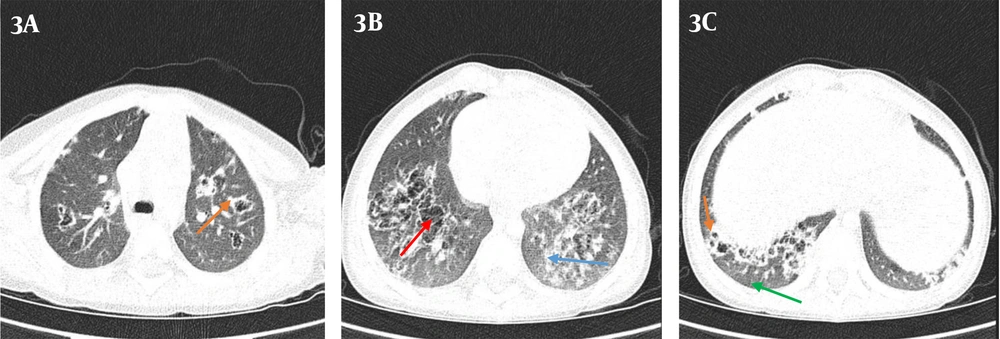
Based on his clubbing, some differential diagnoses such as congenital heart disease and pulmonary impairment were suggested. There was no family history of heart and pulmonary diseases. His intrapartum echocardiography and the newly performed echocardiography were normal, so the congenital heart disease was ruled out. The CXR showed hyperinflated lungs, diffuse bilateral interstitial infiltration, and hazy opacities (Figure 2). Therefore, based on the results of CXR, we requested chest CT scans in which some evidence of recurrent air trapping and also many cavities were detected. In addition, CT scans showed GGO and interlobular septal thickening and multiple lesions and cystic changes with reversed halo sign in both lungs (Figure 3). In this regard, radiologists suggested further follow-ups to determine whether the patient had cystic fibrosis and mucocilliary diseases such as primary ciliary dyskinesia (PCD). Based on the presence of cavities in CT, we looked for the diseases which might cause cavity in lung and also clubbing in the fingers (3, 4). Therefore, we evaluated him for infectious diseases (ie, fungal, viral, and tuberculosis (TB)), rheumatoid diseases (ie, Wegener's granulomatosis and sarcoidosis), gastrointestinal diseases (ie, tracheoesophageal fistula (TEF)), idiopathic pulmonary fibrosis, immunodeficiency diseases (ie, HIV), intrathoracic neoplastic disorders (ie, metastatic cancer, sarcoma, lymphoma, Hodgkin lymphoma, etc.) and suppurative intrathoracic disorders (ie, lung abscess, bronchiectasis, cystic fibrosis).
For the assessment of these diseases, we performed and checked blood tests, urine tests (analysis, culture), purified protein derivative (PPD) skin test, a stool test, throat smear and culture, immunologic factors, barium swallow, CT scans of sinuses (to rule out Wegener’s granulomatosis), abdominal sonography and alpha 1 antitrypsin (AAT) level (Table 1). Negative results of barium swallow, abdominal sonography and also immunologic tests ruled out the some mentioned gastrointestinal, immunologic, and rheumatologic diseases. AAT level was normal, so the AAT deficiency was ruled out. Consequently, the sweat test was performed, and the result was negative, hence cystic fibrosis was ruled out. In these assessments, we found nothing in favor of the mentioned diseases.
| Laboratory Tests | Values |
|---|---|
| Blood test | |
| Complete blood count | |
| White blood cell | 7200 × 103/μL |
| Hemoglobin | 10.5 g/dL |
| Mean corpuscular volume | 71.92 fl |
| Platelet | 382 × 103/μL |
| Differential | |
| Neutrophil | 57% |
| Lymphocyte | 40% |
| Eosinophil | 3% |
| Immunoglobulin (Ig) | |
| IgA | 0.96 g/L |
| IgM | 1.2 g/L |
| IgG | 13.3 g/L |
| IgE | 16.5 U/mL |
| Immunologic factors | |
| CD4 | 32% |
| CD8 | 24% |
| CD16 | 6% |
| CD19 | 33% |
| CD20 | 34% |
| ANA | 1.2 U/mL |
| c-ANCA | 0.9 U/mL |
| p-ANCA | 0.5 U/mL |
| CD56 | 7% |
| Human immunodeficiency virus (HIV) tests | |
| HIV-antibody | Normal range |
| BAL-PCR | HIV-negative |
| Stool exam | Normal flora |
| PPD test | Normal |
| Urine analysis | Normal |
| Urine culture | No growth after 24 h |
| Blood culture | No growth after 72 h |
| BAL-culture | No growth after 72 h |
Abbreviations: CD, cluster of differentiation; ANA, anti-nuclear antibody; ANCA, anti-neutrophil cytoplasmic antibodies; BAL-PCR, bronchoalveolar lavage-polymerase chain reaction; PPD, purified protein derivative.
In the next step, fiber optic bronchoscopy and broncho-alveolar lavage (BAL) were done, and the samples were sent for culture and assessment. Negative results of BAL culture and PPD test ruled out the TB and other fungal and viral infections.
After these steps, the pleural biopsy was performed, and it was discovered that spindle-shaped cells had resulted in the expansion of alveolar walls. Histological findings showed alveolar interstitium with a high level of glycogen-laden mesenchymal cells.
Lung parenchyma demonstrated patchy vimentin-positive interstitial infiltration in favor of interstitial glycogenosis, and therefore, the PIG was diagnosed.
Based on guidelines, we treated this patient with high doses of corticosteroids three days a month, continuing for six months. After that, the serial follow-ups of every six months were carried out.
3. Discussion
In this article, we have presented a case of PIG with an unusual manifestation. The subject was a 1.5-year-old boy and the first and only manifestation of PIG, in this case, was stage 4 clubbing in fingers and toes with no respiratory symptoms of the disease.
PIG is a rare type of ChILD with unknown causes (2, 5). ILD is a heterogeneous group of pulmonary disorders, which is uncommon in the pediatric population (5).
The number of PIG cases that were reported in the articles is less than 100 (5). The presence of concomitant diseases with PIG is common in about 50% of cases of which congenital heart diseases and pulmonary hypertension are the most common (5). In this regard, this case lacked congenital heart disease, pulmonary hypertension, and any other extrapulmonary complications.
PIG is frequently accompanied by bronchopulmonary dysplasia and lung growth abnormalities (2, 6). PIG expresses itself with diverse clinical symptoms such as tachypnea and hypoxia and may lead to acute respiratory failure in neonates (2). In this regard, Canakis et al. reported that all of the seven infants with PIG in their study had tachypnea and hypoxemia in the first month of life (6). In contrast to their findings, pulmonary manifestations were absent in our case.
In another investigation, Liptzin et al. had studied 24 PIG patients. He reported that 71% of patients had pulmonary complications and needed either invasive or non-invasive ventilation, and the incidence of heart disease was 62% in his sample population (1). On the contrary to PIG patients in Liptzin study (1), our case had no pulmonary resuscitation support at birth and no congenital or acquired heart diseases in his echocardiography.
According to the large body of evidence, PIG is a disease of early infancy, and the majority of cases show the disease symptoms in neonatal life (1, 2, 6, 7). In addition, Liptzin et al., in another study, reported that the mean age of PIG diagnosis at the time of lung biopsy was 2.2 months (range: 3 - 6 months) (8). In comparison to these investigations, we diagnosed PIG in our case at the age of 1.5 years, whereas the only presentation was clubbing at fingers and toes, and he did not manifest the pulmonary features of PIG disease and other comorbidities.
Similar to previous articles, we used CT scans and pulmonary biopsy for PIG diagnosis (2, 7, 8). PIG can manifest itself in CT scans through GGO and cystic changes (2), but the diagnosis of PIG can only be made by lung biopsy (5). In this case, after observing the presence of cavities in the CT report, several differential diagnoses were considered, but as described in the section of case presentation, all the assessments about the mentioned differential diagnosis were normal. Other CT findings such as GGO, interlobular septal thickening, and cystic changes were in agreement with CT findings of other PIG cases in the mentioned previous studies (6).
In this case, histological findings in lung biopsy consist of alveolar interstitium with a high level of glycogen in mesenchymal cells, which were consistent with previous studies (6). Lung parenchyma revealed patchy vimentin-positive interstitial infiltration in favor of interstitial glycogenosis. The PIG diagnosis was confirmed by the presence of these biopsy findings (5).
Many studies mentioned that the treatment of PIG with corticosteroids could be beneficial (7). The prognosis of PIG has favorable clinical outcomes if the patient does not have concomitant underlying diseases (7). A study showed that PIG is a disease of interstitial lung cells dysmaturity. The beneficial effects of corticosteroids on PIG are through their effects on dysmaturities rather than on inflammation. Accordingly, we treated this patient with pulses of corticosteroids (6). After that, serial follow-ups were carried out every six months, and no cardiac problems, pulmonary hypertension, and progressive pulmonological complications have been observed. In this article, we presented an educational case without its other common clinical presentations and comorbidities, which could be beneficial in the diagnosis of other cases in the future.