1. Background
In recent decades, preterm delivery and survival of premature infants have increased (1). A recent WHO report shows a 10.6% preterm birth rate (2, 3). The incidence of preterm delivery worldwide is 6.9%, amounting to 12.9 million premature neonates, with the highest rate in Africa and Asia (12 million neonates) (4). Despite the increased survival rate of premature babies, complications continue to rise in these high-risk neonates. Regarding the increased survival of premature neonates, more complications are expected. One of the most critical issues of the care of preterm neonates is the nutrition quality in the first critical days or weeks of life. Inefficient nutrition leads to growth impairment and neurodevelopmental delay in these high-risk neonates (5).
The best source of nutrition for preterm neonates is breast milk. Much research has revealed the beneficial effects of breast milk feeding on neonatal outcomes. The nutrient content of the breast milk of preterm delivered mothers is proportional to the nutritional needs of their children, such as protein content that is higher in premature birth than in term deliveries (6). Given that the mother's nutrition can change the nutrient content of breast milk, many researchers have studied the effects of maternal diet change on neonatal outcomes. Breast milk's long-chain unsaturated fatty acid (LCPUFA) is an essential nutrient required for normal health. Besides, LCPUFA has significant effects on neonates' neurodevelopmental, cognitive, visual, and immunological systems. Docosahexaenoic acid (DHA) is a kind of alpha-linolenic acid (ALA) and omega-3 essential fatty acid with no endogenous production. Breast milk levels of DHA are determined mainly by maternal intake from DHA supplementation (e.g., omega-3 or fish oil supplements) or fish, which can vary markedly around the world (7-9). Thus, maternal nutrition and breast milk are the only exogenous sources of this material in the first months of the life of preterm infants. This valuable nutrient is essential for both mother and child (10). The mean acceptable level of DHA in breast milk is 0.32% of total fatty acids (11).
In some Iranian research, the mean level of LCPUFA has been reported as 3.52 ± 1.4 and 3.8 ± 1.5 g/dL. Increased lactation duration has been associated with increased DHA levels (10-12). The ratio of DHA to total fatty acids is reported as 0.3 ± 0.2% (11). The blood level of DHA in pregnancy is directly related to the gestational age and fetal growth and inversely related to post-delivery anxiety and depression (12). The blood level of neonatal DHA is directly related to the maternal blood and breast milk DHA levels. The neonatal visual function, brain growth, memory, attention, and immunological state strengthen with DHA intake via breast milk. Improved learning and decreased incidence of atopy are the long-term effects of DHA (11).
Seafood contains LCPUFA and DHA, such as Atlantic mackerel (0.59 gm), salmon (1.24 gm), Dicentrarchus labrax (0.47 gm), oyster (0.23 gm), sardine (0.74 gm), shrimp (0.12 gm), and trout (0.44 gm). Oilseeds consisting of linseed, walnut, egg, and fish liver oil have significant amounts of DHA (13-15). In Iran, the most important fish species in the Caspian Sea are Caspian kutum (R. frisii kutum), mullet (L. aurata and L. saliens), and pikeperch (S. lucioperca). Their total PUFA10 content was 23%, 22%, and 20%, respectively (16).
Regarding the low intake of fish products and medicinal supplements containing LCPUFA during pregnancy in Iranian mothers, there is concern about the low level of DHA in breast milk. Thus, measuring breast milk DHA is essential for providing preterm neonates with the best care.
2. Objectives
Given the limited research on the evaluation of DHA in the breast milk of Iranian mothers and regarding the higher probability of neurodevelopmental and growth disorders in preterm neonates, we planned this study to measure the breast milk DHA level and its effects on neonatal outcomes in preterm deliveries.
3. Methods
This descriptive cross-sectional research was done in mothers with preterm delivery in Mahdieh Medical Center affiliated with Shahid Beheshti University of Medical Sciences during 2018 - 2019. We enrolled the mothers of all preterm neonates admitted from 2018 to 2019 with a gestational age of fewer than 36 weeks or a birth weight of lower than 2,000 g. Then, their breast milk was assessed for the DHA level. All mothers had a normal nutritional status and were not underweight or overweight. If neonates were transferred to other medical centers and missed the follow-up sessions, they were excluded from the study. According to Juber et al.'s study (11), 67 mothers with preterm infants were included in our study.
The Ethics Committee of Shahid Beheshti University of Medical Sciences approved our study with the ethical code of IR.SBMU.MSP.REC.1398.745. Written consent was obtained from mothers before taking breast milk samples. To evaluate the DHA level of breast milk, we sampled 5 - 10 milliliters of breast milk and maintained it at 4°C in a refrigerator for up to seven days before transferring it to the laboratory for DHA measurement with gas chromatography. The correlations between the breast milk DHA levels and gestational age, birth weight, gender, delivery mode, supplementation, maternal age, underlying or gestational disorders, maternal intake of DHA-containing food, neonatal outcomes, such as intraventricular hemorrhage (IVH), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), and necrotizing enterocolitis (NEC), and growth indices, including weight gain, increased height, and head circumferences, were evaluated for four months after discharge from the neonatology clinic based on the available growth curve specific for preterm neonates (Fenton growth chart). Four neonates died during hospitalization, and 63 were followed.
Based on Juber et al.'s study (11), the sample size was calculated as 67 patients, with the mean and standard deviation of 0.22 and 0.13%, respectively, and an error level of 0.325.
The mean and standard deviation were used to analyze quantitative variables. Absolute and relative frequencies were calculated for stratified and ranking data. After assessing the normality of variables, the analysis was done by Spearman and Mann-Whitney U tests using SPSS version 25.
4. Results
A total of 67 mothers were eligible for the study. The mean gestational age and birth weights were 31.54 ± 4.44 weeks (25 - 36 weeks) and 1707.85 ± 595.83 g (760 - 3500 g), respectively. There were 38 (56.7%) males and 29 (43.3%) females. The mean maternal age was 28.47 ± 5.42 years (16 - 40 years). The delivery mode was cesarean sections (C/S) in 46 (68.6%) and normal vaginal delivery (NVD) in 21 (31.3%). The mean age of neonates at breast milk sampling was nine days (2 - 44 days). The mean DHA, as a percentage of total breast milk fatty acids, was 0.29 ± 0.0127%. The minimum and maximum levels were 0.09% and 0.73%, respectively.
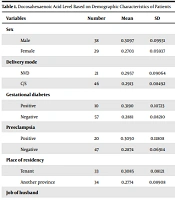
Table 1 shows the mean, minimum, maximum, and median DHA levels of breast milk of mothers based on the demographic data of patients and parents. There was no statistical relationship between all the demographic characteristics of patients and parents and breast milk DHA level (Table 1).
| Variables | Number | Mean | SD | Minimum | Maximum | Median | IQR | P-Value |
|---|---|---|---|---|---|---|---|---|
| Sex | 0.063 | |||||||
| Male | 38 | 0.3097 | 0.09931 | 0.18 | 0.57 | 0.2950 | 0.06 | |
| Female | 29 | 0.2703 | 0.05937 | 0.14 | 0.45 | 0.2700 | 0.14 | |
| Delivery mode | 0.847 | |||||||
| NVD | 21 | 0.2957 | 0.09064 | 0.16 | 0.51 | 0.2600 | 0.09 | |
| C/S | 46 | 0.2913 | 0.08492 | 0.14 | 0.57 | 0.2800 | 0.10 | |
| Gestational diabetes | 0.298 | |||||||
| Positive | 10 | 0.3190 | 0.10723 | 0.18 | 0.57 | 0.3000 | 0.10 | |
| Negative | 57 | 0.2881 | 0.08210 | 0.14 | 0.54 | 0.2800 | 0.13 | |
| Preeclampsia | 0.449 | |||||||
| Positive | 20 | 0.3050 | 0.11808 | 0.14 | 0.57 | 0.2600 | 0.09 | |
| Negative | 47 | 0.2874 | 0.06914 | 0.16 | 0.54 | 0.2800 | 0.12 | |
| Place of residency | 0.140 | |||||||
| Tenant | 33 | 0.3085 | 0.08121 | 0.20 | 0.54 | 0.2900 | 0.10 | |
| Another province | 34 | 0.2774 | 0.08908 | 0.14 | 0.57 | 0.2600 | 0.08 | |
| Job of husband | 0.180 | |||||||
| Self-employment | 30 | 0.2780 | 0.06277 | 0. 14 | 0.40 | 0.2850 | 0.038 | |
| Employee | 17 | 0.2971 | 0.09681 | 0.19 | 0.54 | 0.2800 | 0.06 | |
| Manual worker | 20 | 0.3110 | 0.10548 | 0.20 | 0.57 | 0.2800 | 0.048 | |
| Income | 0.156 | |||||||
| Low | 29 | 0.3072 | 0.09632 | 0.16 | 0.57 | 0.2800 | 0.09 | |
| Intermediate | 37 | 0.2841 | 0.07636 | 0.14 | 0.54 | 0.2800 | 0.12 | |
| High | 1 | 0.1900 | - | 0.19 | 0.19 | 0.1900 | 0.10 | |
| Education of mother | 0.961 | |||||||
| Undergraduate | 16 | 0.3175 | 0.11969 | 0.16 | 0.57 | 0.2800 | 0.15 | |
| Diploma | 26 | 0.2773 | 0.06245 | 0.14 | 0.40 | 0.2750 | 0.10 | |
| Postgraduate | 8 | 0.2588 | 0.05793 | 0.18 | 0.35 | 0.2500 | 0.10 | |
| Bachelor | 12 | 0.3092 | 0.09520 | 0.19 | 0.54 | 0.2900 | 0.06 | |
| Masters | 5 | 0.3080 | 0.07727 | 0.23 | 0.40 | 0.2800 | 0.15 | |
| Education of father | 0.728 | |||||||
| Undergraduate | 19 | 0.2979 | 0.08515 | 0.21 | 0.51 | 0.2800 | 0.09 | |
| Diploma | 25 | 0.2788 | 0.08541 | 0.14 | 0.57 | 0.2800 | 0.08 | |
| Postgraduate | 10 | 0.2850 | 0.05759 | 0.19 | 0.35 | 0.3050 | 0.10 | |
| Bachelor | 8 | 0.3550 | 0.10690 | 0.25 | 0.54 | 0.3400 | 0.18 | |
| Masters | 5 | 0.2580 | 0.08758 | 0.19 | 0.40 | 0.2300 | 0.15 |
Table 2 shows the breast milk DHA level based on the mother's diet and the intake of food rich in this valuable material. Although the nutritional resource of DHA is diverse, consisting of animal and plant bases, it seems that the more intake of food rich in DHA increased the level of this material in breast milk. Fortunately, despite the wide range of maternal diets, the same level of DHA in the breast milk of mothers with different socioeconomic levels was detected. The consumption of seafood (P value = 0.37), omega-6 fish liver oil (P value = 0.93), wheat sprouts (P value = 0.44), walnut (P value = 0.20), nuts (P value = 0.49), soya and cotton (P value = 0.33), canola oil (P value = 0.67), and eggs (P value = 0.94) did not affect the DHA level of breast milk.
| Variables | Number | Mean | SD | Minimum | Maximum | Median | IQR | P-Value |
|---|---|---|---|---|---|---|---|---|
| Use of seafood during last week | 0.377 | |||||||
| Once a week | 44 | 0.2859 | 0.07887 | 0.14 | 0.51 | 0.2750 | 0.095 | |
| Twice a week | 23 | 0.3057 | 0.09903 | 0.16 | 0.57 | 0.2800 | 0.045 | |
| Use of omega-3-6 fish liver oil during last week | 0.936 | |||||||
| Yes | 46 | 0.2933 | 0.08327 | 0.14 | 0.54 | 0.2850 | 0.092 | |
| No | 21 | 0.2914 | 0.09409 | 0.18 | 0.57 | 0.2600 | 0.05 | |
| Wheat sprouts during last week | 0.446 | |||||||
| Yes | 57 | 0.2893 | 0.08844 | 0.14 | 0.57 | 0.2800 | 0.042 | |
| No | 10 | 0.3120 | 0.07208 | 0.21 | 0.45 | 0.3150 | 0.077 | |
| Walnut during last week | 0.201 | |||||||
| Yes | 18 | 0.3150 | 0.10945 | 0.16 | 0.54 | 0.2800 | 0.053 | |
| No | 49 | 0.2845 | 0.07544 | 0.14 | 0.57 | 0.2800 | 0.042 | |
| Nuts during last week | 0.499 | |||||||
| Yes | 23 | 0.3026 | 0.10163 | 0.16 | 0.57 | 0.2800 | 0.04 | |
| No | 44 | 0.2875 | 0.07752 | 0.14 | 0.54 | 0.2850 | 0.05 | |
| Soya and cotton during last week | 0.336 | |||||||
| Yes | 55 | 0.2975 | 0.09054 | 0.16 | 0.57 | 0.2800 | 0.045 | |
| No | 12 | 0.2708 | 0.05992 | 0.14 | 0.34 | 0.2850 | 0.04 | |
| Canola oil during last week | 0.672 | |||||||
| Yes | 61 | 0.2941 | 0.08659 | 0.14 | 0.57 | 0.2800 | 0.05 | |
| No | 6 | 0.2783 | 0.08704 | 0.21 | 0.45 | 0.2450 | 0.045 |
Table 3 shows the incidence of neonatal prematurity complications such as IVH, NEC, BPD, and ROP, neonatal growth indices, and mortality based on the DHA level of breast milk in neonates fed by mothers. There was no significant relationship between the DHA level of breast milk and all neonatal complications. Also, statistical analysis showed no significant relationship between gestational age (P value = 0.32), birth weight (P value = 0.59), sex (P value = 0.1), postnatal age (P value = 0.59), maternal age (P value = 0.93), delivery mode (P value = 0.32), neonatal growth index, and neonatal complications. The relationship between the intake of DHA-containing food and breast milk DHA level was not statistically significant (P value = 0.43).
| Variables | Number | Mean | SD | Minimum | Maximum | Median | IQR | P-Value |
|---|---|---|---|---|---|---|---|---|
| ROP | 0.707 | |||||||
| Negative | 54 | 0.3017 | 0.08975 | 0.14 | 0.57 | 0.2900 | 0.05 | |
| Positive | 13 | 0.2554 | 0.05753 | 0.18 | 0.40 | 0.2500 | 0.037 | |
| BPD | 0.275 | |||||||
| Negative | 52 | 0.2938 | 0.08828 | 0.14 | 0.57 | 0.2800 | 0.05 | |
| Positive | 15 | 0.2887 | 0.08079 | 0.19 | 0.51 | 0.2887 | 0.035 | |
| NEC | 0.588 | |||||||
| Negative | 66 | 0.2935 | 0.08650 | 0.14 | 0.57 | 0.2800 | 0.05 | |
| Positive | 1 | 0.2400 | - | 0.24 | 0.24 | 0.2400 | 0 | |
| IVH | 0.957 | |||||||
| Negative | 55 | 0.2958 | 0.09187 | 0.14 | 0.57 | 0.2800 | 0.05 | |
| Positive | 12 | 0.2783 | 0.05254 | 0.20 | 0.38 | 0.2700 | 0.032 | |
| Appropriate weight gain | 0.856 | |||||||
| No | 56 | 0.2973 | 0.09172 | 0.14 | 0.57 | 0.2850 | 0.05 | |
| Yes | 7 | 0.2500 | 0.02828 | 0.20 | 0.28 | 0.2500 | 0.025 | |
| Appropriate height growth | 0.572 | |||||||
| No | 20 | 0.2838 | 0.06438 | 0.18 | 0.40 | 0.2800 | 0.045 | |
| Yes | 43 | 0.2967 | 0.09473 | 0.14 | 0.57 | 0.2800 | 0.05 | |
| Appropriate head circumference growth | 0.662 | |||||||
| No | 15 | 0.2847 | 0.06032 | 0.19 | 0.40 | 0.042 | ||
| Yes | 48 | 0.2954 | 0.09362 | 0.14 | 0.57 | 0.05 | ||
| Mortality | 0.012 | |||||||
| No | 63 | 0.2940 | 0.10525 | 0.09 | 0.73 | 0.2800 | ||
| Yes | 4 | 0.3300 | 0.04761 | 0.28 | 0.38 | 0.3300 |
The associations between the DHA level and our research variables were evaluated with a crude analysis. Multiple logistic regression modeling adjusted for confounding variables did not show any association between the DHA level of breast milk and neonatal complications. However, these results may be due to the low sample size and a single measurement of DHA level during the study.
5. Discussion
The current descriptive cross-sectional research measured the DHA level in mothers' breast milk with preterm delivery and its effect on common neonatal complications in premature babies. Regarding the impact of maternal diet on the level of free fatty acids such as DHA in breast milk, the measurement of this valuable nutrient in breast milk is important. Changing the maternal or neonatal diet or supplementation resulted in an increasing level of DHA in breast milk. Eventually, this resulted in neonatal circulating with an improvement in the neonatal outcome, especially neurodevelopmental and visual acuity. Despite the infrequent seafood intake in Iranian families and regardless of maternal diet, our research revealed that the mean level of DHA in the breast milk of mothers was in the acceptable range: 0.32% of total fatty acids with the minimum and maximum levels of 0.09% and 0.73%, respectively, in comparison with a worldwide global average level of 0.296 ± 1.27% (17). In research by Fu et al., the mean level of DHA in breast milk was 0.37% of total fatty acids. In this study, Asian mothers had the highest level of DHA, and mothers in low-income countries had a higher level of DHA in breast milk than those in other countries (18).
The level of DHA in breast milk did not reveal statistically significant relationships with birth weight, gestational age, postnatal age, delivery mode, age of mother, socioeconomic level of the family, and common neonatal complications such as BPD, IVH, and NEC. Oxidative stress following normal vaginal delivery changes the level of LCPUFA and DHA in the breast milk of mothers. As the findings of many studies show, the mode of delivery affects the level of breast milk DHA. However, the current study showed no significant difference between NVD and C/S in the level of breast milk DHA (P value = 0.32) (19).
In research by Lapillonne et al., the level of breast milk DHA was decreased during the first month of delivery, and they suggested the fortification of breast milk by DHA supplementation for compensating for this deficiency (20). In our research, the sampling time was different in patients and the mean age of neonates at the time of sampling was nine days (2 - 44 days). Although in our research, there was no significant relationship between the time of sampling and DHA level, similar to the Lapillonne et al.’s study, Martin et al.’s study showed that as the baby grows, the amount of DHA decreases, and the low levels of DHA were associated with the increased rate of BPD. In contrast to research by Smith and Rouse, DHA supplementation raised the rate of BPD in preterm infants (10, 20-22).
The current study showed that newborns with more growth in head circumference, height, and body weight had higher levels of DHA in the breast milk of their mothers, although there was no statistically significant relationship between growth indices and DHA levels. Research by Much revealed that LCPUFA of breast milk improved fat mass growth of infants in the first year of life, and the increased protein and amino acid ratio of breast milk caused better growth indicators (23).
Despite the wide range of maternal diets, the same level of DHA in the breast milk of mothers with different socioeconomic levels was detected. The intake of food rich in DHA did not correlate with the level of DHA in breast milk. Some research revealed the positive effect of breast milk DHA on decreasing BPD incidence in preterm babies (24-26).
The finding of our research revealed the higher growth rate of weight, height, and head circumference in neonates fed by a higher level of DHA in breast milk, although not significantly. In research by Smuts et al. in a clinical trial, supplementation of the maternal diet with DHA during pregnancy made no significant change in the birth weight of neonates (26). The results of Oken et al. and Halldorsson et al. in the United States confirmed the mentioned results. They indicated that supplementation with DHA did not correlate with babies' weight gain and growth index (27, 28).
Contrary to research by Ramakrishnan et al. and Olafsdottir et al., supplementation with DHA improved the fetal weight gain in pregnancy (29, 30). In research by Olsen, the weight gain of neonates with the intake of fish oil was higher than that of the control group (31).
In a study by Davoodabadi Farahani and Seyyed Zadeh Aghdam in Arak, Iran, intending to detect the effect of DHA and eicosapentaenoic acid (EPA) in pregnancy on birth weight and duration of pregnancy, there was a statistically significant difference between mothers receiving DHA supplementation and the control group. They found 284 g reduced birth weight in neonates of mothers but no effect on the duration of pregnancy in the group with DHA supplementation (32). They concluded that this negative effect was due to the difference between monitoring the growth index and the guideline of supplementation with DHA.
The current study did not show any difference in gestational age based on the DHA level of breast milk. In research by De Dooy et al., the levels of breast milk unsaturated fatty acids were increased with increasing gestational age (24).
Two clinical trials revealed the correlation between the omega-3 intake of mothers and the duration of pregnancy so that the duration of pregnancy was prolonged with DHA supplementation in the maternal diet. Olsen et al. detected the longer duration of pregnancy as 2.5% in mothers with fish oil supplementation (26, 31). A systematic review by Kar et al. in 2016 revealed a 58% decrease in preterm deliveries of less than 34 weeks and a statistically significant relationship between gestational age and supplementation with DHA in pregnancy (33). In the research by Olsen et al. and Davoodabadi Farahani and Seyyed Zadeh Aghdam, there was no significant relationship between the duration of pregnancy and DHA, EPA, and fish oil supplementation in pregnancy (31, 32).
The inability of preterm neonates to process fatty acids and produce DHA, the low transplacental transfer rate of this fatty acid from the mother, and low intake of this material after birth due to feeding problems are the concerning issues in this regard (34). The administration of intralipids solutions during NICU admission may be an inadequate source of DHA to meet the baby's needs (21, 35). Regarding the mentioned factors, the enrichment of breast milk with DHA seems logical.
Regarding the contribution of inflammatory reactions as an underlying risk factor for neonatal complications such as NEC, BPD, and ROP, DHA with anti-inflammatory properties can decrease the incidence of these complications of prematurity. A longitudinal study by Martin et al. showed the decreased level of DHA in the first months of life concurrent with the progression and severity of chronic lung disease (21). In research by Martin et al., a 1% decrease in DHA from a total of long-chain fatty acids increased the probability of chronic lung disease by 2.5 times (21). Many published studies confirmed the reverse correlation between DHA levels and incidence of BPD in preterm babies (25, 36).
The findings of a clinical trial by Bernabe‐Garcia et al. in Mexico to evaluate the effect of oral DHA with a dose of 75 mg/kg on the severity of ROP showed the positive prophylactic effect of this material on the incidence and severity of ROP (37). Our research evaluated just the effect of breast milk DHA on neonatal complications, with no pharmacological intervention. Similar to the research of Bernabe-Garcia et al., in research by Bernabe in 110 preterm neonates with a birth weight of 1000 - 1500 g, the incidence and severity of ROP were decreased with DHA supplementation (38). In a clinical trial by Collins et al. in Australia, supplementation with DHA with a dose of 60 mg/kg/day in preterm neonates with gestational age lower than 29 weeks did not improve BPD progression (22).
In a study by Fares et al. in Tunisia, supplementation with DHA decreased mortality related to sepsis, IVH, and respiratory distress syndrome (39). However, our research did not show any positive effect of breast milk DHA on neonatal mortality. The single laboratory measurement of this nutrient of breast milk is the most significant limitation of our research, which limited the study results.
The limitations of our research were the low sample size of the study, financial constraints (due to the high cost of laboratory exams), and the measurement of breast milk DHA just in one sample of breast milk and at different times after delivery (2 - 44 days post-delivery). For more conclusive results, larger studies with more sample size and repeated measurements of breast milk DHA or supplementation of the maternal or neonatal diet with this valuable nutrient is recommended.
The current study's findings showed the acceptable levels of DHA in the breast milk of mothers with preterm delivery, although it did not reveal a significant correlation between maternal and neonatal factors and neonatal outcomes. The low sample size and evaluation of just one breast milk sample can justify no significant effects observed. Regarding the well-known beneficial effects of breast milk, research with a higher sample size and repeated measurements of DHA is recommended.