1. Background
Neonatal sepsis (NS) is a major cause of death in newborns, mainly because it may result in significant consequences. Hence, a low threshold should be used to evaluate or treat these patients (1). NS is a systemic problem caused by bacterial, viral, or fungal agents, which may cause hemodynamic alternation in newborns younger than 28 days. Sepsis includes the isolation of an agent from body fluid normally sterile like blood or cerebrospinal fluid (CSF). Nevertheless, as a clinical property of sepsis, it may be stimulated by potent pro-inflammatory cytokines. The term systemic inflammatory response syndrome (SIRS) has also been considered for describing NS (2).
The sequential organ failure assessment (SOFA) score can predict death and high-risk intensive care unit (ICU) admissions. Organ ailment reveals more multiplex pathobiology compared to sole infection with an inflammatory reaction (3). The categorization of sepsis is based on the age of infants, as well as the age of diagnosing symptoms. Early-onset sepsis (EOS) is defined as the presentation of signs before the first week of life, though some experts restricted the definition to infections diagnosed at the first three days of life (2). Late-onset sepsis (LOS) is defined as the presentation of signs after the first week of life. Various definitions are provided, ranging from the first three days of life to more than a week (4). EOS develops before birth (in utero) from either transplacental or ascending bacteria due to the rupture of the membrane, which is more frequent (5, 6). The incidence of NS varies from one to five per 1000 live births. In 2013, NS and other important infections Accounted for nearly 430,000 lives worldwide, which is nearly 15% of total neonatal mortality (7-9). Group B Streptococcus (GBS) and Escherichia coli are reported as the main reasons for EOS and LOS, which in total account for nearly 66% of EOS cases (10). More frequent viral reasons for sepsis include the herpes simplex virus, which leads to considerable morbidity and mortality. Viral infections may cause symptoms that are similar to sepsis and can be concentrated on the skin, eyes, and mouth, or may affect the central nervous system (CNS), which may initiate in 5 to 9 days of life (11, 12). The major neonatal parameter inclining to infection which may cause sepsis is prematurity or low weights at birth that is associated with increased risk of infections (by 3 to 10 times) compared to full-term infants with a normal weight (13). Maternal risk factors are related to the enhanced probability of sepsis, especially GBS infection (2), which contains chorioamnionitis, respiratory problems, or ingestion of bacteria in amniotic fluid and may result in congenital respiratory problems or severe infection (1), intrapartum maternal temperature ≥ 38°C (100.4ºF), delivery at < 37 weeks of gestation, maternal GBS colonization, membrane rupture ≥ 18 h. The risk of confirmed sepsis enhances by 10 times to 1% in cases with ruptured membrane for more than 18 hours (14). Regarding the importance of the type of delivery in the development of neonatal sepsis, the main germs in vaginal delivery were Escherichia coli, Group B streptococcus, Staphylococcus epidermis, Staphylococcus aureus, and Clostridium, but Staphylococcus aureus and Staphylococcus epidermis were more common in cesarean section (15).
Clinical symptoms span from subtle signs to considerable septic disturbances due to the subtleness of clinical manifestations of sepsis. Hence, it is vital to identify neonates at increased risk of NS and those with an increased suspicion for sepsis among those who deviated from the usual pattern of activity or feeding (1). Hematological criteria like the total number of white blood cells (WBC), absolute neutrophil count (ANC), absolute band count (ABC), immature to total white blood cell ratio (I/T ratio), and platelet count are widely applied for the assessment of NS (16). More recently, advanced WBC criteria, including mean neutrophil volume (MNV), mean monocyte volume (MMV), neutrophil distribution width (NDW), and monocyte distribution width (MDW) were introduced as new markers of NS (17). WBC < 5000 to 7500/mm3 is the criterion for NS diagnosis. The diagnostic biomarkers of NS necessary for early identification of patients before the occurrence of symptoms are investigated and identified (18, 19).
2. Objectives
This study aimed to evaluate the NLR and PLR as diagnostic adjunct tests for EOS.
3. Methods
This prospective cross-sectional study was conducted in the Minia University Hospital, Egypt, from April 2018 to April 2019. Using admission logs, a total of 867 neonates born at 37 weeks of gestational age (GA) were identified. There were 92 patients with conventional risk factors and clinical presentations for NS. Eight patients did not meet the inclusion criteria, and four patients declined to participate in the study. Finally, 80 patients were enrolled in the study. Also, 80 healthy neonates were considered as the control group. The patients with NS were selected according to the standard risk factors and clinical presentations (neonatal-specific SOFA) (3). Based on the ultra-sonographic examinations and the novel Ballard Scoring.
System. The inclusion criteria were neonates born by natural delivery or Cesarean-section (C-section) with 37 to 42 weeks of GA and a confirmed diagnosis of sepsis. The exclusion criteria were: multiple pregnancies, pre-or post-maturity, small for gestational age (SGA), large for gestational age (LGA), preeclampsia, gestational diabetes mellitus (GDM), chorioamnionitis, history of smoking during pregnancy, congenital problems, cyanotic congenital cardiovascular problem, negative C-reactive protein (CRP), and procalcitonin. Both groups were matched in terms of age, gender, and health status.
3.1. Clinical and Laboratory Assessments
Gender, birth height (BW), birth head circumference (HC), weeks of gestation (WG), Apgar scores at 1 and 5 minutes after delivery, and delivery mode were all reported in the data. All examinations were done within 24 hours of the birth. In the first 72 hours after delivery, NS was defined as a positive blood culture combined with infection symptoms (eg, respiratory distress, apnea, cyanosis, etc.). Negative blood, urine, and CSF cultures were considered for NS. The significant symptoms of infection and positive diagnostic factors included an immature to total neutrophil ratio [I/T ratio] > 0.2, total leukocytes [WBCs] of either 5109/L or > 15109/L, thrombocytopenia [150,000/mm3], CRP level more than 1 mg/dL, and procalcitonin level > 0.5 ng/mL). Meningitis was diagnosed from high leukocyte count (> 20/mm3) and high protein concentration (> 150 mg/dL) in the CSF and bacterial growth in the CSF culture (11-13). Total blood count contained hemoglobin, RBCS, red cell indices (MCV, MCH, and MCHC), platelet count, and WBS count (both total and differential). It was evaluated by an automated hematology analyzer, Celltac G (Nihon Kohden Corporation). Differential leucocyte count was approved using microscopic assessment of Lishman stain blood film, and the immature/total neutrophil ratio (I/T) was calculated. The neutrophil/lymphocyte ratio was used to calculate NLR, and the platelet/lymphocyte ratio was used to calculate PLR. The ESR was calculated using the Westergren method. CRP levels were also determined using the immunoturbidimetric method, and procalcitonin levels were determined using the electrochemiluminescence immunoassay (ECLIA) method (Roche Cobas 6000; Roche Diagnostics GmbH, Mannheim, Germany).
3.2. Sample Size
The sample size was calculated using a single population proportion formula, with the proportion obtained from previous research. According to a previous study conducted in Egypt, EOS was the most common type of sepsis (31.8%) (3). The sample size was estimated using a 95% confidence interval (CI) and a % marginal error:
n = required sample size; z = the standard normal deviation at 95% confidence interval = 1.96; p = proportion of neonatal sepsis among neonates admitted in NICU with the prevalence of 44.7; d = margin of error that can be tolerated, 5% (0.05); 1-p = proportion of the population that do not possess the character of interest.
Our overall sample size was 160 neonates, with a non-response rate of 5%. The correction formula was employed, and the final sample size was 160 neonates. The study population consisted of all neonates admitted to governmental neonatal intensive care units (NICUs) in the area. After determining the number of hospital admissions in the previous year, the number of research subjects for the hospital was determined. The study subjects were chosen using a systematic random sampling method (every Kth) after calculating the "Kth" value by dividing the total number of neonates born in the previous year by the required sample size, which was allocated proportionally to each hospital, and the first subject was chosen by lottery method. Medical records with all relevant information were used.
3.3. Data Analysis
Data were analyzed in SPSS software version 20 using descriptive (mean and standard deviation) and analytical statistics. Also, the t-test, Mann–Whitney U test, chi-square test, Fisher’s exact test (FET), and correlation analysis were employed for statistical analysis. Statistical significance was considered as P-value < 0.05. Quantitative variables were tested for normality using the Kolmogorov–Smirnov test. The sensitivity, specificity, and positive and negative predictive values of NLR and PLR were calculated using the area under the receiver-operating characteristic (AUROC) curve.
4. Results
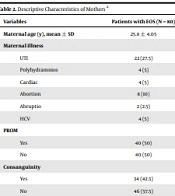
The demographic information and maternal data of both study groups were provided in Tables 1 and 2. According to the findings, the premature rupture of membrane (PROM) was a significant maternal risk factor found in 50% of participants. In addition, we found considerable reasons for maternal diseases between the two groups, concerning variables like urinary tract infection (UTI), polyhydramnios, cardiac problems, abortion, and hepatitis C virus (HCV).
| Patients with EOS (N = 80) | Controls (N = 80) | P-Value | |
|---|---|---|---|
| Age (d) | 0.2 | ||
| Mean ± SD | 2.25 ± 0.9 | 2 ± 0.9 | |
| Median | 3 | 2 | |
| Weeks of gestation | 0.7 | ||
| Mean ± SD | 38.7 ± 0.2 | 38.8 ± 0.7 | |
| Median | 38.06 | 39.63 | |
| Birth weight (kg) | 0.1 | ||
| Mean ± SD | 2.9 ± 0.2 | 3 ± 0.2 | |
| Median | 2.9 | 3 | |
| Maternal age (y) | 0.9 | ||
| Mean ± SD | 25.8 ± 4 | 25.7 ± 4.1 | |
| Median | 26.5 | 26 | |
| Apgar score (min) | 0.0001* | ||
| 1st | |||
| Mean ± SD | 5.2 ± 0.8 | 4.5 ± 12.2 | |
| Median | 5 | 9 | |
| 5th | 0.09 | ||
| Mean ± SD | 8.9 ± 0.3 | 9.07 ± 0.3 | |
| Median | 9 | 9 | |
| Consanguinity | 0.01* | ||
| Positive | 34 (42.5%) | 14 (17.5%) | |
| Negative | 46 (57.5%) | 66 (82.5%) |
The Demographic Characteristics of Patients with EOS and Controls a
| Variables | Patients with EOS (N = 80) |
|---|---|
| Maternal age (y), mean ± SD | 25.8 ± 4.05 |
| Maternal illness | |
| UTI | 22 (27.5) |
| Polyhydramnios | 4 (5) |
| Cardiac | 4 (5) |
| Abortion | 8 (10) |
| Abruptio | 2 (2.5) |
| HCV | 4 (5) |
| PROM | |
| Yes | 40 (50) |
| No | 40 (50) |
| Consanguinity | |
| Yes | 34 (42.5) |
| No | 46 (57.5) |
Descriptive Characteristics of Mothers a
Distribution of cases by major presenting complaints is described in Table 3. Out of 80 full-term neonates affected by NOS, refusal of feeding (ROF) was the major complaint. Besides, 90% (n = 72) of participants had respiratory distress, while 65% (n = 58) had weak peripheral perfusion and shock. The markers of participants, separated by the study groups, are presented in Table 4. Leucocyte count was considerably higher in neonates with NS than controls (P = 0.004). Besides, neonates with NS had a significantly lower platelet count and lymphocytes than controls (P = 0.04). Also, they experienced a considerable enhancement in immature neutrophil count and I/T ratio compared to the controls (P = 0.0001). Comparison of NLR and PLR among the study groups revealed significantly higher values of these ratios in neonates with NS compared to controls, which indicates their major contribution in the identification of EOS (P = 0.0001). Moreover, neonates with NS had a considerably higher CRP level and procalcitonin compared to controls (P = 0.0001). According to the findings, out of 80 participants, Klebsiella was the most common (50%) gram-negative organism causing NS isolated from blood culture (Table 5). In addition, at the threshold of 0.1, the sensitivity and specificity of NLR were 67% and 99%, respectively. Also, for the threshold of 7, the sensitivity and specificity of PLR were 70% and 73%, respectively. Besides, for the threshold of 4.7, the sensitivity and specificity of CRP were 80% and 70%, respectively, while at the threshold of 85.5, the sensitivity and specificity of procalcitonin were 82% and 90%, respectively. In addition, NLR had a PPV of 98%, and PLR had a PPV of 72% (Table 6).
| Signs | No. (%) |
|---|---|
| Respiratory | |
| RD | 58 (72.5) |
| Cyanosis | 16 (20) |
| Apnea | 6 (7.5) |
| Feeding | |
| ROF | 72 (90) |
| Fair | 4 (5) |
| Vomiting | 2 (2.5) |
| Intolerance | 2 (2.5) |
| Neurological | |
| DCL | 44 (55) |
| Seizures | 24 (30) |
| Fair neurological | 12 (15) |
| Cardio-circulatory (peripheral perfusion, shock) | |
| Poor | 52 (65) |
| Fair | 28 (35) |
| GIT | |
| Edema | 58 (72.5) |
| Hepatomegaly | 22 (27.5) |
Distribution of Cases by Major Presenting Complaints
| Cases (N = 80) | Controls (N = 80) | P-Value | |
|---|---|---|---|
| WBCs | 0.004* | ||
| Mean ± SD | 17 ± 9.3 | 11.9 ± 4.7 | |
| Median | 15.5 | 11.7 | |
| Interquartile range | 10.2 - 22.4 | 8 - 15.8 | |
| Platelets | 0.04* | ||
| Mean ± SD | 193 ± 84 | 227.5 ± 78.4 | |
| Median | 174.5 | 234 | |
| Interquartile range | 136 - 257.5 | 162.5 - 303.2 | |
| Immature neutrophils | 0.0001* | ||
| Mean ± SD | 13.6 ± 19 | 2.8 ± 12.3 | |
| Median | 8 | 0 | |
| Interquartile range | 0 - 20.3 | 0 - 0 | |
| I/T | 0.0001* | ||
| Mean ± SD | 0.5 - 0.9 | 0.05 - 0.2 | |
| Median | 0.1 | 0 | |
| Interquartile range | 0 - 0.5 | 0–0 | |
| Lymphocytes | 0.0001* | ||
| Mean ± SD | 23.2 ± 15 | 37.9 ± 15.5 | |
| Median | 18.5 | 35.5 | |
| Interquartile range | 12.2 - 34.7 | 25 - 51.5 | |
| CRP | 0.0001* | ||
| Mean ± SD | 14 - 13.4 | 8.1 ± 13.7 | |
| Median | 7.7 | 2.8 | |
| Interquartile range | 4.9 - 17.9 | 1.3 - 8.6 | |
| Procalcitonin | 0.0001* | ||
| Mean ± SD | 139.1 ± 66.7 | 53.8 ± 26 | |
| Median | 126.5 | 54.5 | |
| Interquartile range | 97 - 157 | 35.2 - 76 | |
| NLR | 0.0001* | ||
| Mean ± SD | 0.8 ± 1.1 | 0.08 ± 0.3 | |
| Median | 0.4 | 0 | |
| Interquartile range | 0 - 1.1 | 0 - 0 | |
| PLR | 0.0001* | ||
| Mean ± SD | 15 ± 12.4 | 5.9 ± 3.5 | |
| Median | 9.7 | 5.5 | |
| Interquartile range | 6.2 - 22 | 3.09 - 7.5 |
Markers in Patients with EOS and Controls
| Blood Culture | No. (%) |
|---|---|
| Klebsiella | 40 (50) |
| Candida albicans | 6 (7.5) |
| Candida-klebsiella | 2 (2.5) |
| Staph haemolyticus | 14 (17.5) |
| Streptococcus pneumonia | 2 (2.5) |
| MRSA | 2 (2.5) |
| MRSE | 4 (5) |
| Enterococcus | 4 (5) |
| CoNS | 6 (7.5) |
List of Pathogens Identified in Positive Blood Cultures
| Cut-off Value | AUC | 95% CI | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
|---|---|---|---|---|---|---|---|
| NLR | 0.1 | 0.79 | 0.68 - 0.89 | 67 | 99 | 98 | 75 |
| PLR | 7 | 0.78 | 0.68 - 0.88 | 70 | 73 | 72 | 71 |
| CRP | 4.7 | 0.76 | 0.65 - 0.87 | 80 | 70 | 72 | 77 |
| Procalcitonin | 85.5 | 0.92 | 0.86 - 0.98 | 82 | 90 | 89 | 83 |
NLR and PLR as Predictors of Sepsis
5. Discussion
The neonatal period is a crucial stage in a human’s life. It is linked to significant physical and cognitive developmental abnormalities in neonates. The diagnosis of neonatal sepsis is typically complex and time-consuming. The etiology of this problem stems from the difficulty in distinguishing its clinical signs from those of other newborn disorders. Blood or cerebrospinal fluid cultures are the gold standards for diagnosing newborn sepsis, especially bacterial sepsis. However, it is time-consuming, which may cause the treatment to be postponed and result in extensive dissemination of the pathogenic organism pathogenic organisms (20). Every year, almost four million newborn fatalities are reported around the world. Sepsis is responsible for one-third of these deaths. Bacterial meningitis and sepsis are the leading causes of newborn death, especially in neonates with very low birth weight (VLBW). To avoid significant and life-threatening complications, newborn sepsis must be identified and treated promptly (21). Neutrophils are triggered during sepsis or tissue infection, causing them to rise (22). Neutrophils are the most frequent leukocyte (> 50% of leukocytes). Besides, they are experts at phagocytosis and destroying microbes (23). Circulating platelet–neutrophil complexes contain a wide spectrum of inflammatory ailments and sepsis. Stimulated platelets can attach to neutrophils and mediate neutrophil recruitment to damage and infection areas (24). Although WBC is a routine diagnostic technique for sepsis examinations, it is widely accepted as a well-founded criterion of infection. However, it is both insensitive and nonspecific. Moreover, a sole leukocyte count shortly after the delivery is not sufficiently sensitive for the diagnosis of NS (25).
The current research aimed to evaluate the PLR and the NLR and to estimate their value as diagnostic markers for the identification of EOS in full-term neonates. According to our findings, neonates with NS had a considerably higher leukocyte count compared to controls, which indicates the major contribution of leukocytosis in the diagnosis of NS (P = 0.004). This is inconsistent with the results of studies carried out by Can et al. (26) and Xiao et al. (27). Moreover, Jefferies (28) found that a low WBC was more likely to be associated with EOS than high TLC. In addition, neonates with NS had a lower platelet count and lymphocytes compared to controls, which was correlated with thrombocytopenia and neonatal death as a major consequence of NS (P = 0.04). In concordance with our results, in a study by Can et al. (26), the lymphocyte count in neonates with NS was considerably lower than controls. This difference in neutrophil and lymphocyte count between patients and controls can be explained by the fact that the natural immunological responses of circulating leukocytes to a variety of stressful situations are characterized by a higher neutrophil count and a decreased lymphocyte count. A microorganism infection causes an inflammatory reaction, which results in increased total leukocyte and neutrophil numbers (29, 30). In addition, those with sepsis experienced a considerable enhancement in immature neutrophil count and I/T ratio than controls, which reveals the crucial role of CBC with a differential in the identification of EOS (P = 0.0001); this was also proven by Cekmez et al. (31). Our results were not in line with those of Can et al. (26), that reported no statistically significant differences in the I/T ratio between the EOS group and the control group. A comparison of NLR and PLR between the study groups revealed considerably greater values in the sepsis group compared to the controls, indicating that these ratios play a significant role in the detection of EOS (P = 0.0001). In the current study, there was a statistically significant increase in the NLR between the patients and controls, which is similar to the studies by Can et al. (26), Omran et al. (32), and Wilar (33), reporting that NLR was significantly higher in the patients compared to the controls.
Regarding PLR, our results showed a significant increase among patients compared to controls. This is in agreement with the study by Can et al. (26), reporting that PLR was significantly higher in the EOS group. On the other hand, Omran et al. (32) found no statistically significant difference between the PLR of EOS group and controls. Our results showed a statistically significant increase in CRP levels between patients and controls, which is in agreement with the studies by Can et al. (26), Omran et al. (32), and Xiao et al. (27). Also, it is in line with the studies conducted by Sorsa (25) and Hotoura et al. (34), who reported CRP as an important diagnostic method for EOS, and its maximum is during the first two days with higher sensitivity and specificity. In addition, Albrich and Harbarth (35), Gilfillan and Bhandari (36), Ng et al. (37), and Franz et al. (38) mentioned that diagnostic precision of CRP can be enhanced using the combination of biomarkers like interleukins or procalcitonin. This study demonstrated the significance of procalcitonin, as an alternative biomarker, to CRP in identification of EOS, which is in line with the study by Chiesa et al. (39). The neonates in the sepsis group showed significantly higher procalcitonin levels compared to the controls. This agrees with the study by Joram et al. (40), Mithal et al. (41), and Steinberger et al. (42), who found that the procalcitonin level was significantly higher in the sepsis group compared to the non-septic group. Furthermore, in two recent studies by Can et al. (26) and Rashwan et al. (43) the procalcitonin level was significantly higher in sepsis group than controls. In the present study, gram-negative bacilli, such as Klebsiella, accounted for 50% of the organisms identified, whereas gram-positive cocci, such as Staph haemolyticus, accounted for 17.5 percent of culture-proven sepsis. This is in line with a study by Patel et al. (44), Sharma et al. (45), and Vaniya et al. (46), who found that gram-negative bacilli were the most common organisms, mainly Klebsiella. However, other studies stated that gram-positive bacteria, mainly staphylococci account for the majority of the culture growth (47-49).
Lee et al. (50) also reported that gram-positive organisms were the most predominant organisms of EOS in South Korea. This difference in isolated organisms shows that every neonatal unit has its own pattern of microorganisms, which change from time to time, and antimicrobial combinations should be altered according to culture results. Can et al. (26) found that NLR of 6.76, which was determined as the predictive cut-off value of neonatal EOS, had a sensitivity of 97.4 percent and a specificity of 100 percent at a cut-off point of 0.1, while NLR of 6.76, which was determined as the predictive cut-off value of neonatal EOS, had a sensitivity of 97.4 percent and a specificity of 100 percent at a cut-off point of (32). On the other hand, Omran et al. (32) observed that NLR at a cut-off point of 2.7 presented 80% sensitivity and 57.1% specificity. Moreover, Wilar (33) found that NLR at the cut-off point of 1.42 showed 83.3% sensitivity and 93.3% specificity.
Can et al. (26) found that the value of neonatal EOS had a sensitivity of 97.4 percent and a specificity of 100%, at a cut-off point of 7. PLR had a sensitivity of 70% and a specificity of 73% at a cut-off point of 7. This difference is due to the absence of an accurate cut-off point for PLR in EOS, and there is not enough research on this issue. In our study, procalcitonin showed a sensitivity of 82% and a specificity of 90% at a cut-off point of 85.5 ng/mL, while Pontrelli et al. (51) reported that procalcitonin showed a sensitivity of 85% and specificity of 54% at a cut-off point 2.0 ng/mL.
In a systematic review and meta-analysis by Chiesa et al. (39), the authors studied the procalcitonin accuracy in neonates from 1998 - 2014 using the standards for reporting of diagnostic accuracy (STARD) initiative; they found that procalcitonin sensitivity ranged from 47.4 to 100% and specificity from 35.3 to 100%.
In our study, the I/T ratio showed a sensitivity of 78.8% and a specificity of 92% in the diagnosis of EOS. However, Saboohi et al. (52) reported that the I/T ratio showed a sensitivity of 76.47% and a specificity of 83.82% in the diagnosis of EOS.
In the current study, CRP level showed a sensitivity of 80% and a specificity of 70% in diagnosis of EOS. However, Hisamuddin et al. (53) reported that CRP level showed a sensitivity of 76.9% and a specificity of 53.49% in the diagnosis of EOS. Moreover, Naser et al. (54) reported that CRP level showed a sensitivity of 90.32% and specificity of 42.10% in the diagnosis of EOS.
5.1. Limitations
This study had some limitations. First, our study included only appropriate for gestational age (AGA) term neonates. Because these diseases were linked to early period neutrophil and platelet counts in newborns, premature neonates, SGA and LGA neonates with GDM, and maternal chorioamnionitis neonates were excluded. Second, generalizing our results should be done with caution due to the small sample size.
5.2. Conclusions
NLR and PLR are valid predictive markers for early identification of NS as the PPV of NLR and PLR was 98% and 72%, respectively. Based on laboratory investigations, leukocytosis, thrombocytopenia, high CRP, high procalcitonin, and positive blood culture were correlated with the risk of NS. Accordingly, NLR, PLR, I/T ratio, serum CRP, and procalcitonin levels can be employed as diagnostic adjunct tests for identifying EOS in term AGA neonates. However, further large multicenter trials with larger sample sizes encompassing all categories of newborns are required.