1. Introduction
Coarctation of the aorta (CoA) is a congenital heart defect. Due to the narrowing of the descending aorta, blood flow mainly reduces after the stenosis, and CoA can occur at any region in the thoracic and abdominal aorta. This problem can occur alone or with other heart lesions, such as state aortic valve, ventricular septal defect, patent ductus arteriosus, dextro-transposition of the great arteries, and atrioventricular septal defect (1, 2). The CoA accounts for about 6 - 8% of all congenital heart defects, which is about 4 per 10,000 live births and is more common in males than females (3). Children with CoA might experience various symptoms, including absence or weakness of the femoral pulse, delayed capillary refill, differences between the femoral and radial pulses, higher blood pressure, and saturation of upper extremity compared to the lower one, nutritional problems, hypotonia, and metabolic acidosis (4).
The CoA is currently repaired through three methods including surgical procedure (i.e., stenotic aortic resection and end-to-end aortic anastomosis), transcatheter balloon angioplasty, and transcatheter stent implantation (4), which among them, in neonates and children with severe CoA, surgery is commonly considered the first-line intervention (5). However, the surgery is associated with postoperative complications, such as CoA recurrence, aneurysm formation, stable hypertension (6), and cerebral infarction in very rare cases. The present study reviewed the cases of three patients with cerebral infarction developed after CoA repair surgery in a period of 5 years. The present case report was based on the CAse REport (CARE) guidelines (7).
2. Case Presentation
2.1. Case 1
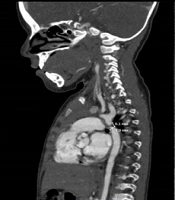
An 18-day-old male patient with a 3 kg weight, 50 cm height, and 34 cm head circumference was transferred from a peripheral hospital to the Vali-e-Asr Hospital in Birjand, Iran, with a bad general condition. In the initial examinations, the patient had tachypnea and tachycardia, and the blood pressure difference between the upper and lower extremities was 20 mmHg. He had metabolic acidosis, creatinine of 0.9 mg/dL, and the right hand and left leg oxygen saturation (SpO2) of 95% and 88%, respectively. Electrocardiogram (ECG) examination showed signs of left ventricular hypertrophy, and mild cardiomegaly was noted on the chest X-Ray. A murmur (2.6) was heard on the chest with maximum intensity between two scapulae. A diagnosis of CoA and an atrial septal defect (ASD) was made for the patient, and treatment with prostaglandin E1 0.2 µg/kg/minute started after echocardiography.
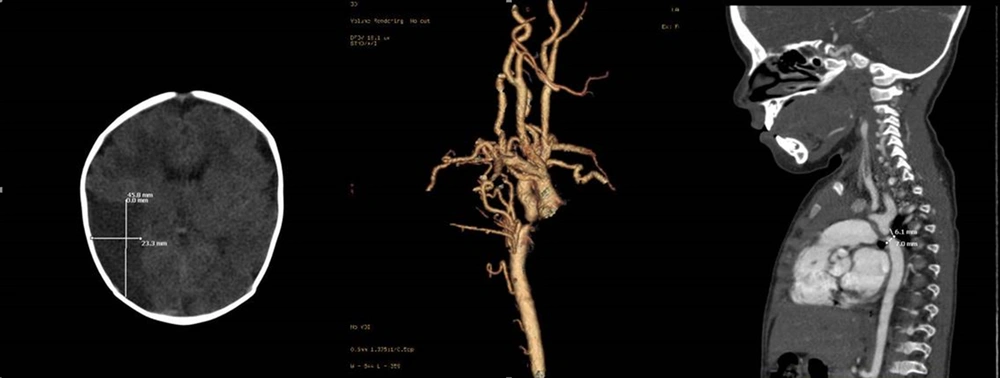
After partial recovery, emergency aortic surgery was performed by the complete resection of the stenosis in the aorta and end-to-end anastomosis of the two remaining arteries. One day after surgery and extubation, the patient developed choreoathetosis attacks, seizures, and restlessness, controlled with phenobarbital therapy. On brain computed tomography (CT) scan, a hypodense lesion was observed in the right frontoparietal lobe (Figure 1). In the first 48 hours after the operation, the patient’s blood pressure was controlled with hydralazine and Inderal, and the pulse status and the blood pressure difference between the upper and lower extremities were desirable after surgery and pharmacotherapy.
Following the recovery of vital signs and cessation of seizures, the patient was discharged after 3 weeks with the prescription of phenobarbital and was called for follow-up at 1, 3, 6, 12, and 24-month intervals. Based on echocardiographic findings, there was no gradient at the surgical site and no pressure difference between the four limbs; therefore, phenobarbital was discontinued after 3 months. The evolutionary process was accompanied by delays in neck holding up, sitting, and walking; consequently, neurological practices, including occupational therapy and speech therapy, were taken, and choreoathetosis symptoms improved dramatically. After 3 years of follow-up, the patient finally was able to walk, hold up his neck, sit, and talk with a delay. The evolution continued with a slight delay in comparison to his peers with no other complication.
2.2. Case 2
The patient was a 7-year-old child with 17 kg weight, 95 cm height, and 48 cm head circumference who had undergone stenting at the CoA site following the diagnosis of isolated CoA in infancy. The case was referred to Vali-Asr Hospital for hypertension and re-coarctation of the aorta (reCoA) on serial echocardiography reports. After initial examinations by echocardiography and angiography, 60 and 50 mmHg gradient differences were observed before and after stent site and a 30 mmHg blood pressure difference between the upper and lower extremities. Physical examinations indicated a femoral pulse delay with no differences in the pulse oximetry of the upper to lower extremities and a murmur (2.6) between the two scapulae. Electrocardiographic signs were in favor of left ventricular hypertrophy, and cardiomegaly and left ventricular hypertrophy were also observed in chest radiographs.
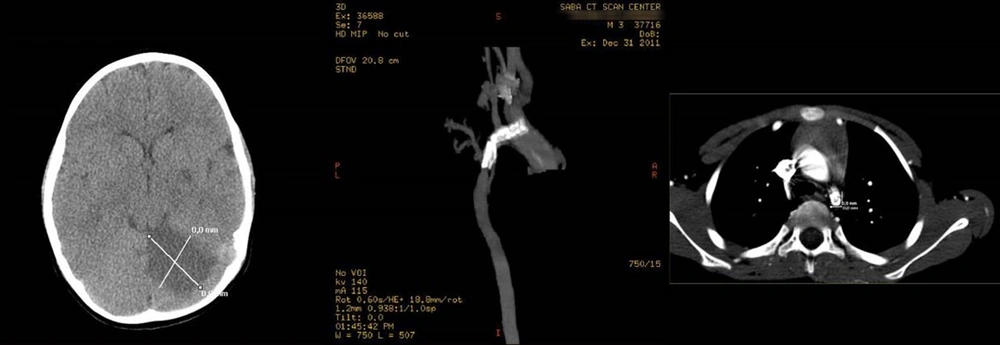
Due to the standard weight and stable vital signs, the patient was referred to the surgical ward for reCoA total correction, end-to-end aortic anastomosis, and stent removal. Three days after successful surgery, a brain CT scan was performed due to seizures, headache, and visual disturbances, in which an ischemic infarction was observed in the occipital area (Figure 2). The patient was treated with dexamethasone, mannitol, phenobarbital, and physiotherapy in consultation with the department of pediatric neurology. In the first 48 hours after the operation, the patient’s blood pressure crisis was controlled with captopril and Inderal; nonetheless, the pulse status and blood pressure differences between the upper and lower extremities were favorable.
The patient was discharged from the hospital after 4 weeks following the recovery of vital signs and cessation of seizures; accordingly, dexamethasone and mannitol administration was stopped, and phenobarbital was prescribed. The case was called for follow-up at 1, 3, 6, 12, and 24-month intervals. Based on echocardiographic findings, there was no CoA gradient and blood pressure and no differences between upper and lower extremities; therefore, phenobarbital was discontinued after 3 months. Following consultation with the pediatric neurology department, visual disturbances showed improvement in 12- and 24-month follow-ups, and other therapeutic practices, including physiotherapy and occupational therapy, improved the patient’s motor signs.
2.3. Case 3
A 28-day-old neonate was admitted to a neonatal intensive care unit of Vali-Asr Hospital, with respiratory distress and tachypnea during the coronavirus disease 2019 (COVID-19) pandemic. A murmur (3.6) was heard in the interscapular space. Furthermore, the reduced SpO2 of the lower extremity compared to the upper one and a 20 mmHg blood pressure difference between the extremities were evident. A chest X-ray showed cardiomegaly with perihilar infiltration and lung periphery, and left ventricular hypertrophy was also apparent on ECG. Then, echocardiography was performed for the neonate; accordingly, preductal CoA was diagnosed. Laboratory test results showed metabolic acidosis, which was controlled. The neonate was treated with prostaglandin E1 and referred to the department of pediatric cardiac surgery.
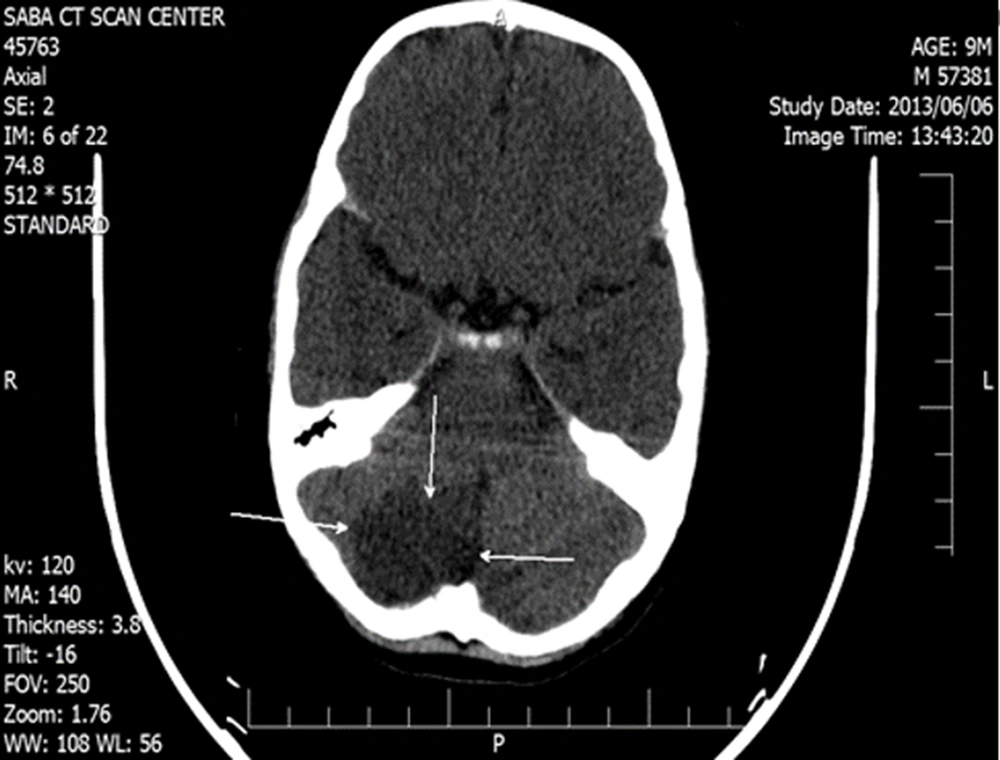
The neonate underwent CoA repair surgery by the complete resection of the aortic stenosis and anastomosis of end-to-end arteries. After 6 hours of extubation, the patient was transferred to the ward in a good general condition, and breastfeeding started. However, 48 hours after surgery, decreased consciousness, hypotonia, and seizure were reported in the patient; accordingly, a posterior cerebrovascular accident (CVA) was detected in the brain CT scan (Figure 3). At the same time, his polymerase chain reaction test result for COVID-19 was also positive. The patient was treated with phenobarbital and levetiracetam. In 1, 3, 6, 12, and 24-month follow-ups, phenobarbital and levebel were discontinued within 3 months. No significant developmental delay was observed at 6- and 12-month examinations in comparison to those of the peers. It was impossible to differentiate the cause of CVA in this patient due to CoA repair from COVID-19.
3. Discussion
In the 1950s, CoA repair surgeries in neonates and children increased widely, following the introduction of prostaglandin E2 in the mid-1970s, to treat critically ill neonates with complex heart disease and stabilize their condition; however, neonates and children undergoing CoA repair surgery might experience complications (8) due to a variety of factors, such as anatomical abnormalities of the aortic arch, additional heart defects, and commodities and the experience of the surgical team and the surgeon (9).
In a study performed by Lerberg et al., relied on a 25-year experience in CoA repair surgery in 334 neonatal and pediatric patients, several postoperative complications were reported which the most common complications were paradoxical hypertension, pulmonary complications (e.g., atelectasis, pleural effusion, respiratory failure, and simple pneumothorax), and infection (10). Numerous studies also reported other complications, such as mesenteric arthritis syndrome, spinal cord paralysis, chylothorax following rupture of the lymphatic duct after CoA repair, diaphragmatic paralysis, restenosis of the repair site, and rebound hypertension (11-15). However, in the meantime, cerebrovascular complications are observed in patients after CoA repairs due to various causes, such as defects in the aortic valve and cerebral arteries, especially the circle of Willis and vertebral artery hypoplasia, or the employed method of repair (e.g., end-to-end anastomosis or the use of a subclavicular flap) (16, 17). We did not have this complication in the form of transcatheter balloon angioplasty and transcatheter stent implantation.
Cerebral hemorrhage can occur for various reasons, such as aneurysms or the persistence of hypertension, even after CoA repair. Regarding the occurrence of stroke after CoA repair, there is a hypothesis, based on which after surgery and CoA repair and the nature of the disease, the brain parenchymal tissue is exposed to a lot of blood flow. This increases intracranial pressure after pathological correction because the blood flow to the brain tissue decreases suddenly, which in intracranial pressure causes cerebral ischemia (17). It seems that the cases reported in the present study developed heart attacks for similar reasons. On the other hand, cerebral infarction in two of the reported cases occurred in the occipital lobe. According to the evidence, vertebral artery hypoplasia is a risk factor for infarction in the posterior lobe of the brain and cerebellum; it might also be the cause of infarction in the posterior cerebral artery (18). It should be noted that in the third case, cerebral infarction occurred simultaneously with CoA repair surgery and COVID-19 infection; the differentiation of the cause of the lesion was difficult (19).
In a long-term study performed by Felling et al. on patients with CoA undergoing repair surgery in childhood, the chance of developing a stroke was higher than their healthy peers in adulthood, which strengthens the hypothesis of cerebrovascular disorders in such patients; this point is also highlighted in the patients of the present study (20).
The prevalence of postoperative infarction is rare among children; however, it is of particular importance because they are often neglected due to the need for the early repair of the lesion and the inability to express the neurological symptoms of a stroke. Therefore, pediatric cardiologists and pediatric heart surgeons are recommended to consider any suspicious neurological symptoms seriously and pay attention to them in CoA treatment in children. Performing cerebral CT angiography or magnetic resonance angiography and magnetic resonance venography before surgery might be better to rule out aneurysms and possible cerebrovascular defects in neonates and children. It is also suggested to perform a review study if some case reports are available, summarize the results and possible causes, and formulate and test a hypothesis in an animal house to rule out incorrect causes.