1. Background
Acute diarrhea in children with an annual prevalence of about 2 billion episodes accounts for 1.9 million deaths in developing countries (1). Acute diarrhea is defined as three or more episodes of loose and watery stools daily for three days or more and less than 14 days (2). Symptoms of acute diarrhea include loose or watery stool, which can be accompanied with hypoxia, vomiting, and abdominal pain. Moreover, acute watery diarrhea is still leading to significantly high morbidities in infants and toddlers (3, 4). Those suffering from comorbidity or severe malnutrition require special measurements since they are prone to complications such as electrolyte disturbances, infections, and death (5). Among children below five years old, 516 deaths on average have been annually reported to be caused by acute diarrhea in Iran (6).
The characteristics of diarrhea (e.g., watery, mucous, or bloody stools), duration of symptoms, history of travel, past medical history (immunosuppressives or comorbidities), vaccination, drug history (e.g., antibiotics or laxatives), family history of similar manifestations, and also the child’s weight before the presence of diarrhea should be reviewed (7).
The pathogenesis of acute diarrhea in children is possible by two different mechanisms, namely secretory or osmotic. In general, treatment in children is underpinned by supportive care with rehydration to counteract the intestinal loss of water and electrolytes and also the prescription of zinc supplements (8).
According to the WHO, only 35% of children with diarrhea receive proper treatment for dehydration; hence, the detection of appropriate interventions enables us to prevent mortality and decrease the morbidity rate. Global goals are to reduce mortality in children below five years old to one or less in 1,000 live births and decrease the annual incidence of severe diarrhea to 75% by 2025 (9).
Regarding the WHO’s strategies, the goals of treatment are to improve the care and referral system to manage facilities in health centers and provide proper supplements, ORS, zinc, antibiotics if needed, oxygen in children below five years old, and breastfeeding in infants (9).
Although diseases leading to acute diarrheal are often self-limiting, shortening the duration of acute diarrhea would bring about significant benefits. Although oral rehydration therapy (ORT) decreases mortality, it does not decrease the period of diarrhea or the number of defecations per day (3).
Recently, new drugs have been introduced to help physicians manage acute diarrhea, such as Racecadotril as an enkephalin inhibitor (10) and a prodrug. Thiorphan is an active metabolite of Racecadotril, which selectively stops the activity of the neutral endopeptidase (NEP). This endopeptidase is introduced on the epithelial cells of the small intestine and the renal epithelium (11, 12).
Enkephalins inhibit the production of adenosine monophosphate cyclic in the gastrointestinal system and decrease the hypersecretion of water and electrolyte (11). NEP degrades the enkephalins. The secretion of intestinal enterotoxins is stimulated by cAMP (13). Finally, the antisecretory influence of Racecadotril is induced by the NEP inhibition, thereby reducing the secretion of water and electrolyte in the small bowel tract (11, 13). This would gradually lead to a decrease in watery and loose stool (14). Unwanted effects such as severe constipation, abdominal pain, or abdominal distension have not been reported (10).
Two randomized clinical trials have evaluated the impact of racecadotril on the time scale of diarrheal episodes. These studies revealed that the duration of diarrhea decreased in the racecadotril group compared to those prescribed a placebo or nothing (15, 16). Three analyses on the medical reports of children from the UK, Thailand, and Malaysia claimed that racecadotril + ORS was more advantageous and cost-benefit than ORS therapy, resulting in saving up to 900 Euros/patient in the health care systems (17).
Clinical research addressing the cons and pros of racecadotril in the management of acute diarrhea has ended up with inconsistent results. While some studies concluded an incredible decrease in stool volume, the number of defecations, and the period of gastroenteritis, some other studies reported no specific merit (18-21).
2. Objectives
The present study aimed to evaluate the effect of Racecatodril in children with gastroenteritis. This research aimed to compare the effect of mixed therapy (Racecadotril and ORS) with ORS therapy in children with gastroenteritis admitted to hospitals.
3. Methods
This prospective randomized clinical trial was conducted from September 2018 to May 2019 in the Gastroenterology Ward of a national referral pediatrics center at the Mofid Children’s Hospital. The participants were assigned to the control ORS and treatment (Oral Rehydration Solution + Racecadotril) groups. The zinc supplement was also prescribed for both groups.
Children aged 3 - 60 months with acute diarrhea were included in this study. The exclusion criteria were children with dysentery or mucus in stool, children with severe comorbidities such as liver or kidney diseases, and a history of specific drug ingestion or food allergies.
Children meeting the inclusion criteria were randomly assigned into two groups and received either standard treatment or racecadotril plus standard treatment. The study was approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences (Code: IR.SBMU.REC.1395.1) and registered with the Iranian Registry of Clinical Trials (IRCTID: IRCT201607131264N8).
Racecadotril (Zedott, Torrent Pharmaceuticals Ltd, Gujarat, India) was prescribed (1.5 mg/kg every eight hours). Racecadotril administration lasted until improvement of diarrhea symptoms or five days after the start of the treatment. Apart from additional therapy by racecadotril, all children received the medications according to the WHO’s recommendations.
One hundred children were included in the present study, of whom five persons were excluded: Two persons in the control group due to the loss of follow-up information, two persons in the Racecadotril group due to parental request, and one person due to eligibility violation.
The randomization was performed with a 1:1 allocation ratio. Of 100 participants, the research protocol could be conducted for 95 children. Among the participants, 52 out of 54 children were administered oral rehydration solution (ORS), and 43 out of 46 children received ORS+Racecadotril according to the recommendations of the Centers for Disease Control and Prevention (22, 23).
During the initial visit, the baseline demographic variables, including age, gender, and weight (kg), were collected, and the participants’ body weight, pulse rate, respiratory rate, temperature, and dehydration severity were assessed. All children hospitalized with acute non-invasive gastroenteritis were divided into two groups: a group with severe dehydration and another group with moderate dehydration.
Acute non-invasive gastroenteritis was proved by a standard stool exam and stool culture (First stool specimen was taken at the hospital and analyzed in terms of bacterial infections using standard microbiologic methods).
On daily follow-up visits after admission, parents or caregivers were asked about several daily bowel movements, stool consistency (based on the Bristol stool scale (24) in Table 1), fever, nausea, vomiting, and any new symptoms or side-effects. Children discharged before the complete resolution of symptomswere followed up by phone interviews.
| Score | Description |
|---|---|
| 1 | Separate hard humps |
| 2 | Sausage-shaped but lumpy |
| 3 | Sausage-shaped with cracks |
| 4 | Sausage or snake-like, smooth and soft |
| 5 | Soft blobs with clear-cut edges |
| 6 | Fluffy pieces with ragged edges, mushy stool |
| 7 | Watery, no solid pieces, entirely liquid |
The Bristol Stool Scale
The present study mainly aimed to evaluate the effectiveness of the prescription of Racecadotril based on the count of bowel movements and the recovery rate 24 and 48 hours after the treatment initiation. The secondary objective of this study was to determine changes in the duration of diarrhea, the consistency of stool, and the safety and tolerability of Racecadotril.
All data were classified in a pre-prepared questionnaire and were divided into quantitative and qualitative variables. The collected data was then analyzed. First, the distribution normality of the studied quantitative variables was confirmed using the Kolmogorov-Smirnov test. Then, the chi-squared test was used to compare the qualitative variables between the groups. To investigate the effect of each independent variable, we calculated 95% confidence intervals. The analyses were performed using SPSS software version 25.0. In this study, P < 0.05 was set as the significance level for all tests.
4. Results
Ninety-five children with acute diarrhea who met the inclusion criteria participated in this study. The mean age of children was 14.8 (± 8.9 SD) months in the ORS group and 13.7 (± 10.9 SD) months in the ORS+R group. In the ORS+R group, 52% of the patients were male, and 48% were female. In the ORS group, 70% of the patients were male, and 30% were female.
According to statistical analyses, there was no significant difference between the racecadotril and the control groups in terms of age and gender (P = 0.182 and P = 00.093, respectively).
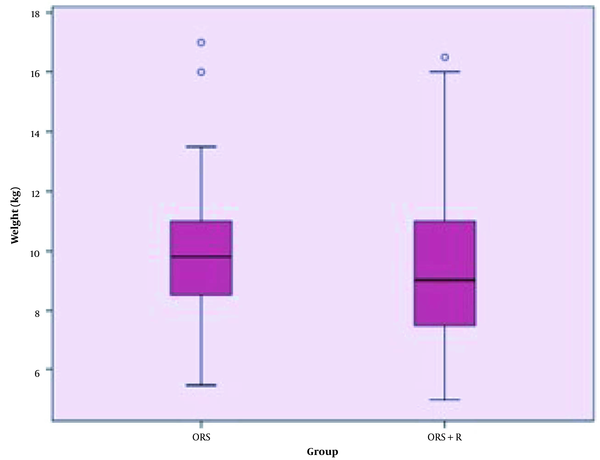
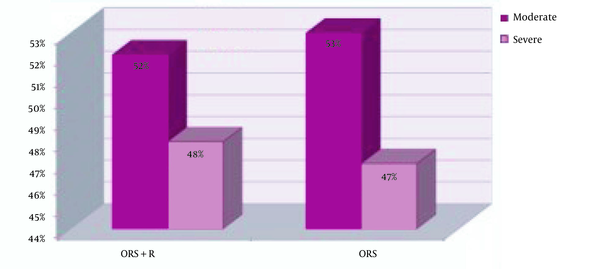
The mean weight of children in the intervention and control groups were not significantly different (P = 00.506) (Figure 1). Moderate dehydration was reported in 22 (52.4%) children in the ORS+Racecadotril group and 28 (52.8%) children in the ORS group. Moreover, dehydration status was severe in 20 (47.6%) children in the ORS+racecadotril group and 25 (47.2%) children in the control group. Statistically, the dehydration severity was similar in both groups (P = 01.0) (Figure 2).
Comparison of dehydration severity between the control and Racecadotril groups. In the ORS+R group, there were 22 patients (52.4%) with moderate dehydration and 20 patients (47.6%) with severe dehydration, while in the ORS group, there were 28 patients (52.8%) with moderate dehydration and 25 patients (47.2%) with severe dehydration. The difference between the groups was not statistically significant regarding dehydration levels (P = 1.0).
At the time of admission, 30 children (71.4%) had vomiting, and 19 patients (45.2%) had a fever in the ORS+R group, while in the ORS group, 42 patients (79.2%) had vomiting, and 24 patients (45.3%) had fevers. Our statistical analyses showed no meaningful difference between the ORS+R and ORS groups regarding fevers and vomiting (P = 1.0 and P = 0.471, respectively). All laboratory findings at the time of admission were similar in the two groups (Table 2).
| Lab Variables | Treatment Groups | P-Value | |||
|---|---|---|---|---|---|
| ORS+R | ORS | ||||
| Mean ± SD | Range | Mean ± SD | Range | ||
| Potassium (mEq/L) | 4.2 ± 0.5 | 3 - 5.7 | 4.4 ± 1.0 | 3.2 - 9.0 | 0.946 |
| Sodium (mEq/L) | 139.3 ± 4.6 | 130 - 149 | 139.2 ± 5.9 | 132 - 160 | 0.739 |
| Creatinine (mg/dL) | 0.7 ± 0.1 | 0.5 - 0.9 | 0.7 ± 0.2 | 0.5 - 1.4 | 0.640 |
| BUN (mg/dL) | 14.3 ± 6.9 | 4.4 - 38 | 15.7 ± 8.8 | 4.4 - 42 | 0.622 |
| Neutrophil (%) | 50.7 ± 17.7 | 20 - 83 | 49.9 ± 21.1 | 13 - 90 | 0.857 |
Laboratory Findings at the Time of Admission a
The average period of diarrhea before admission was estimated to be about 1.8 days (± 1.3 SD) in the group receiving ORS and 2.0 days (± 1.6 SD) in the group receiving mixed therapy (P = 0.96). The average period of diarrhea after treatment initiation was longer in the control group (3.9 ± 1.6 SD days) than in the Racecadotril group (3.0 days ± 1.4 SD) (P = 0.005). However, statistical analyses showed no meaningful difference between the groups regarding the total duration of diarrhea (P = 0.78).
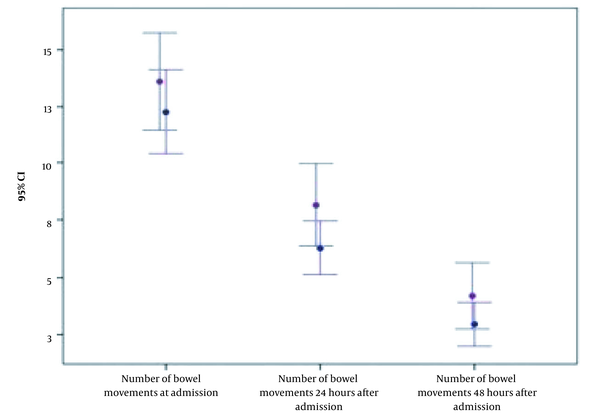
No remarkable difference was noticed in the mean of bowel movements at the beginning of the study (12.0 (± 6.7 SD) in the ORS group vs. 11.2 (± 6.1 SD) in the ORS+R group (P = 0.847). After 24 hours, the mean of bowel movements reduced to 6.9 (± 5.6 SD) in the control group and 4.6 (± 3.2 SD) in the ORS+Racecadotril group. After 48 hours, the frequency of bowel movements decreased to 4.5 (± 4.5 SD) in the control group and 3.0 (± 2.4 SD) in the racecadotril group. Figure 3 shows the rapid decline in the frequency of bowel movements during the first and second days after the initiation of treatment in both groups; however, no statistically significant difference is presented (P = 0.079 and P = 0.221, respectively).
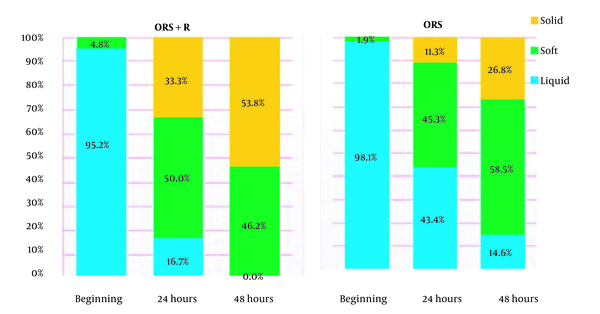
According to statistical analyses, there was no remarkable difference between the two groups at the time of admission in terms of stool consistency (P = 0.58). Moreover, 24 and 48 hours after treatment, the decrease in the number of loose stools and the increase in solid defecations were more significant in the racecadotril group than in the control group (P = 0.005 and P = 0.025, respectively).
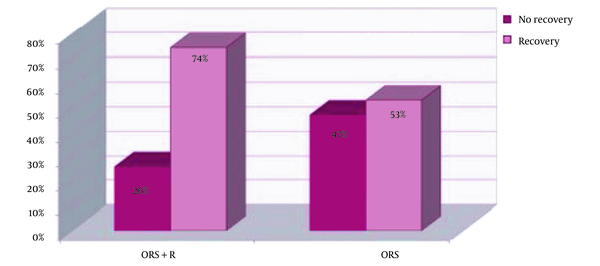
After 24 hours of treatment, 41 children (77.4%) in the ORS group and 27 children (64.3%) in the ORS+R group had diarrhea. The analytical results indicated no statistically significant difference between the two groups regarding the recovery rate during the first 24 hours of treatment (P = 0.177). Two days after treatment, 11 children (26.2%) in the Racecadotril group and 25 children (47.2%) in the control group still had diarrhea. The 48-hour recovery rates were 74% in the ORS+R group and 53% in the ORS group. Although the recovery rate of children treated for 48 hours was larger in the racecadotril+ORS group than in the control group, no significant difference was observed (P = 0.055).
It should be noted that no side-effect was reported during the study. To sum up, there was no statistically meaningful difference between the intervention and the control groups in terms of age, gender, and weight. Statistically, the dehydration severity was the same in both groups. Moreover, no statistically significant difference was found between the two groups regarding fevers and vomiting. All laboratory findings at the time of admission were similar in both groups. Although the average period of gasteroenteritis was shorter in the racecadotril group than in the control group, there was no statistically significant difference between the two groups. Our findings showed a rapid decline in the number of defecations in the first and second days after the initiation of treatment in both groups; however, there was no statistically meaningful difference. The results showed no statistical difference in recovery rates between the two groups in the first 24 hours of treatment. Moreover, although the recovery rates of children treated for 48 hours were larger in the racecadotril+ORS group, no significant difference was revealed.
5. Discussion
We studied the effectiveness of racecadotril in children younger than five years old with acute diarrhea who were admitted to hospitals. Although several relevant studies have been conducted in different countries, there has been no published data on racecadotril in Iran.
The present study dealt with children aged 3 - 60 months with acute gastroenteritis who required hospitalization. The dehydration severity was similar in both groups.
According to the findings, that racecadotril did not significantly reduce the number of bowel movements in 24 hours (6.9 ± 5.6 bowel movements in the ORS group vs. 4.6 ± 3.2 bowel movements in the ORS+R group, P = 0.079) and 48 hours (4.5 ± 4.5 bowel movements in the ORS group vs. 3.0 ± 2.4 bowel movements in the ORS+R group, P = 0 .221).
Santos et al.’s study on 189 patients showed no difference in the mean duration of diarrhea and the frequency of bowel movements between the two groups two days after the therapy (16). This similarity of results may be due to the similarity of samples in terms of dehydration severity and other symptoms. This is, while the sample size should also be considered because larger sample sizes would provide more reliable statistical relationships.
Fecal consistency (a decrease in the number of watery and loose defecations and an increase in solid defecations) was improved in the ORS+ R group in the first and second days after the initiation of the therapy. Kang et al. found no advantage in racecadotril treatment in terms of the period of diarrhea, the volume of stool, the number of defecations, or water and electrolyte loss requirements (25).
In contrast, Salazar-Lindo et al. showed that the two-day stool output, total stool, and the mean duration of diarrhea were less remarkable in the racecadotril group than in the placebo group (26). This could be caused by minor differences in study design and even differences in drug prescription protocols.
Many studies have examined the effect of racecadotril in the management of acute gastroenteritis in children; however, the findings are inconsistent. Our study showed that racecadotril causes a decline in the duration of diarrhea after hospitalization. Furthermore, the recovery rate was higher in children receiving racecadotril 24 and 48 hours after initiating treatment; however, the statistical analyses revealed no meaningful difference. Michael et al. reported that total 48-hour stool output, the number of bowel movements, and the period of gastroenteritis were less significant in the racecadotril+ORS group than in the control group (27). Moreover, a recent systematic review claimed that racecadotril had moderate effectiveness in curing children with diarrhea compared to other anti-diarrheal treatments (28). On the other hand, a meta-analysis by Eberlin et al. concluded that racecadotril was more effective than other medications in managing acute gastroenteritis in children (29).
Different studies have reached different findings, which may be due to different mechanisms of diarrhea. Secretory diarrhea persists during fasting, while osmotic diarrhea occurs after ingesting poorly-absorbed solutes (30). However, the effectiveness of racecadotril in managing acute gastroenteritis is based on inhibiting the activity of enkephalinases; therefore, the final outcome is to decrease the secretion of water and electrolytes with no change in the motility of the bowel (13, 31). Eventually, racecadotril, which stops the process of secretory diarrhea, had no pleasing effect on osmotic diarrhea. In other words, it may be effective for some types of diarrhea but not be for others, depending on the different mechanisms leading to diarrhea. Accordingly, further studies should be conducted to differentiate the types of diarrhea in children and compare the effects of racecadotril on each type.
Since racecadotril does not alter intestinal motility, unwanted effects such as abdominal pain, abdominal distention, and rebound constipation are not supposed to happen (32). The present study demonstrated that racecadotril was a safe medication for children with acute diarrhea, and that the incidence of vomiting was similar in both groups. Other studies have also confirmed that racecadotril is well-tolerated in children (25, 27, 33).
The main limitation of this study was the small sample size, which was due to the hospital refers and compliance. Accordingly, further studies are suggested to include larger sample sizes in several hospitals to achieve more accurate results.
Since acute diarrhea is a self-limiting disease, the disease duration from the beginning of the treatment to recovery may be the most applicable and relevant parameter from patients’ or their parents’ perspectives. Accordingly, the study results on the diarrhea duration may somehow depend on the patients' or their parents' concepts.
5.1. Conclusions
According to the findings, there was no significant relationship between racecadotril administration with the duration of diarrhea, the frequency of diarrhea, and the recovery rate on the first and second days after therapy. However, in the first and second days after therapy, there were a decrease in the number of watery stools and an increase in solid stools; hence, further studies with larger sample sizes and more accurate measurements determining factors affecting acute diarrhea and differentiating different types of diarrhea are recommended to further illustrate the role of racecadotril on the treatment of diarrhea in children.