1. Background
Umbilical cord connects the fetus with placenta. It is a vital structure. Umbilical cord development began to start around third week of gestation and completely develop at seven weeks (1). Umbilical cord thickness depends on lumen of vessels and Wharton’s jelly (2, 3). The principle function of umbilical cord is keeping the cord vessels. These vessels exchange the blood between placenta and fetus and help in growth of fetus (4). The Umbilical cord diameter affects the overall outcome of fetus (5). Wharton's jelly is an extracellular matrix, gelatinous material. It protects the umbilical cord vessels from compression or bending (6). Intrauterine growth retardation (IUGR) predisposes by Reduction in wall thickness of the umbilical cord arteries and vein (7). Thus, the overall thickness of umbilical cord is contributed by its vessels, Wharton’s jelly. So, it can be concluded that reduction in umbilical cord thickness and diameter can compromises the fetal growth. Reduced fetal growth has its own implications on immediate postnatal and long-term neonatal outcomes.
Neonatal sepsis is the main causes of death in neonates. Various infections can be the origin of sepsis and infected umbilical cord is one of the sources of sepsis that can lead to cellulitis, omphalitis and eventually sepsis (8, 9). Bathing of neonates after birth does not affect the rate and type of infection of umbilical cord. But, early separation of cord was seen in non-bathing babies (10).
In spite of valuable effort of medical health services, there are various antenatal maternal risk factors affecting the health of newborn. Antenatal maternal risk factors are defined as maternal risk factors that adversely affecting the pregnancy and health of fetus are known as antenatal maternal risk factor. Severe anaemia, hypothyroidism, Gestational diabetes, Malpresentation, twin or multiple pregnancy, placenta previa, Bad obstetric history, hypertensive disorder of pregnancy are consider as high risk factors in pregnancy (11-14). Following antenatal maternal risk factors (Bad obstetric history, Blood transfusion during pregnancy, History of Pregnancy induced hypertension, Gestational diabetes mellitus, Hypothyroidism, Oligohydramnios, Meconium-stained liquor, Prolonged rupture of membrane, Malpresentation, Anaemia in mother) were studied and correlated with neonatal placental thickness (Table 1). So, the maternal first antenatal care visit completed by evaluation of maternal total cholesterol, high-density lipoprotein and low-density lipoprotein. These blood parameters could influence the outcome of umbilical cord length, diameter, and area (5).
| Parameters | Number of Neonates | Umbilical Corddiameter | P-Value |
|---|---|---|---|
| Bad obstetric history | 0.77 | ||
| Yes | 4 | 10.54125 ± 2.055425 | |
| No | 299 | 10.30832 ± 2.396631 | |
| Blood transfusion during pregnancy | 0.27 | ||
| Yes | 22 | 9.81364 ± 2.708103 | |
| No | 281 | 10.35036 ± 2.363914 | |
| History of pregnancy induced hypertension | 0.92 | ||
| Yes | 26 | 10.07442 ± 3.218658 | |
| No | 277 | 10.33364 ± 2.303087 | |
| Gestational diabetes mellitus | 0.39 | ||
| Yes | 5 | 9.55800 ± 1.965205 | |
| No | 298 | 10.32403 ± 2.396841 | |
| Hypothyroidism | 0.12 | ||
| Yes | 8 | 8.90063 ± 2.399012 | |
| No | 295 | 10.34965 ± 2.381842 | |
| Oligohydramnios | < 0.01 | ||
| Severe | 9 | 8.01389 ± 1.496234 | |
| Moderate | 7 | 9.35429 ± 1.830286 | |
| Mild | 7 | 9.23143 ± 2.571269 | |
| No | 280 | 10.43617 ± 2.380049 | |
| Meconium-stained liquor | < 0.01 | ||
| Yes | 26 | 8.864 ± 2.381 | |
| No | 277 | 10.445 ± 2.350 | |
| Prolonged rupture of membrane | 0.07 | ||
| Yes | 12 | 9.07208 ± 3.144532 | |
| No | 291 | 10.36250 ± 2.346379 | |
| Malpresentation | 0.67 | ||
| Yes | 14 | 10.37643 ± 2.337042 | |
| No | 289 | 10.30824 ± 2.396096 | |
| Anaemia in mother | 0.17 | ||
| Yes | 28 | 9.56179 ± 2.724864 | |
| No | 163 | 10.42900 ± 2.394953 |
a Values are expressed as mean ± SD.
2. Objectives
- To evaluate the umbilical cord thickness in newborn;
- To identified the antenatal maternal risk factors in study population;
- To determine the correlation between umbilical cord thickness and antenatal maternal risk factors.
3. Methods
3.1. Study Design
this is a cross sectional prospective study which was conducted in the neonatal intensive care unit of a tertiary care center in north India. This study conducted to collect data (umbilical cord thickness, antenatal history, after delivery follow-up of newborns). This study started from august 2020 to July 2021 at Rajkiya Mahila Chikitsalaya, Jawahar Lal Nehru Medical College, Ajmer, India.
3.2. Study Population
We enrolled 303 neonates for this study. One hundred and fourteen consecutive neonates born from mother completed gestation period 34 weeks and newborn birth weight > 1250 grams. Mother of These newborns had antenatal maternal risk factors. Mothers of other 189 newborns were free from antenatal maternal risk factors and considered as control group. In this group mother also completed gestation period 34 weeks and newborn birth weight > 1250 grams.
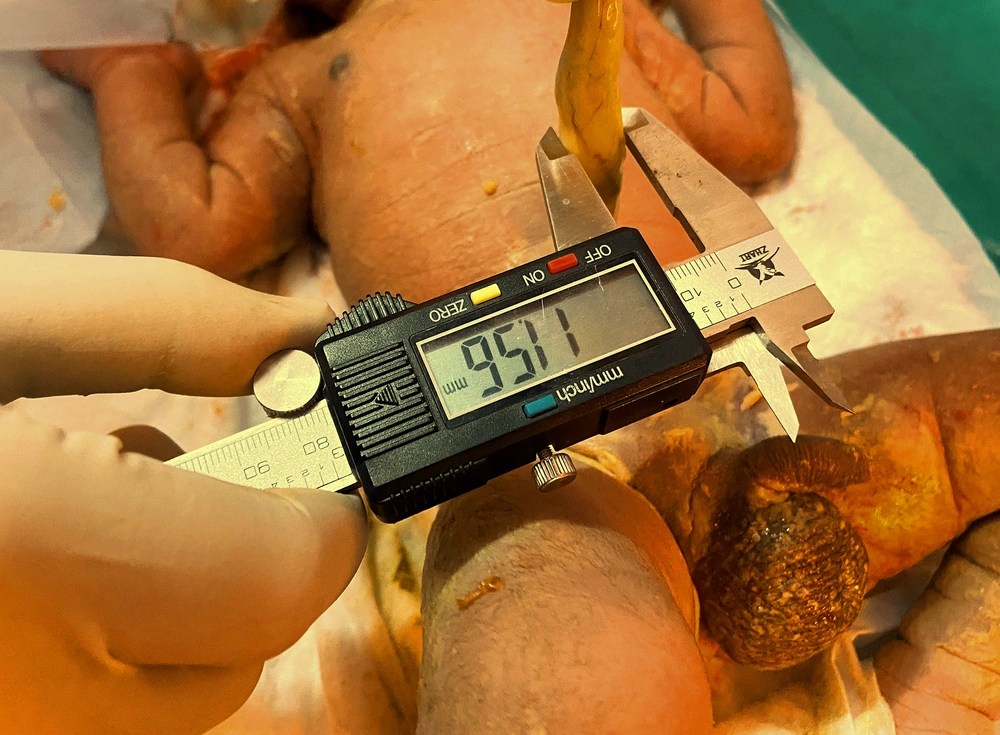
All patients were subjected to a protocol (as per proforma) which included a detailed clinical history of antenatal maternal risk factors, relevant examination, birth weight, Apgar score and meconium staining recorded. After delivery, umbilical cord diameter was measured for all neonates at 2.5 cm above the base of cord at neonatal side by digital vernier caliper (accuracy up to 0.01 mm) (Figure 1).
Digital vernier caliper used for this study – Brand – Q fun, material stainless steel.
3.3. Inclusion Criteria
- Neonates associated with antenatal risk factor was considered as case.
- Neonates who are not associated with antenatal risk factor was considered as control.
- Single pregnancy.
3.4. Exclusion Criteria
- Newborns having weight < 1250 gram;
- Newborn born before 34 weeks of Gestational age;
- Neonates with IUGR, low birth weight were excluded.
3.5. Study Size
We collect the sample as our convenience and simple.
3.6. Statistical Analysis
The data was analyzed using Statistical Package for Social Sciences (SPSS) Version 23. Proportions were compared using chi-square test while mean values were compared using Independent‘t’ test. Multiple linear regression analysis was done to see the association of different risk factors with umbilical cord diameters. The confidence limit of the study was kept at 95% hence a P-value less than 0.05 was considered statistically significant.
4. Results
Three hundred three newborns were found suitable according to inclusion criteria during study period. We found mean gestation age was 37 ± 2.1 weeks in the study population. Mean gestational age at delivery was significantly lower in mother with antenatal risk factor as compared to those without antenatal risk factors (37 ± 2.1 week versus 38.14 ± 1.64 week; P = 0.01). There was preponderance of male newborn in cases (55%) while gender distribution in controls was equal. Mean umbilical cord diameter was smaller in newborns with antenatal risk factors as compared to newborns without antenatal risk factors (cases: 9.89 ± 2.53 mm; controls: 10.56 ± 2.26 mm) and the difference was statistically significant (P = 0.03).
Oligohydramnios and meconium-stained liquor were found to be associated with the smaller umbilical cord diameter (P < 0.01) (Table 1). With increase in severity of oligohydramnios from mild to moderate to severe, the umbilical cord diameter was found to be significantly smaller (P < 0.01). There was no significant correlation between maternal age and gestation with umbilical cord diameter (Table 2).
| Variables | Values |
|---|---|
| Maternal age | |
| r-value | 0.016 |
| P-value | 0.804 |
| No. | 245 |
| Gestation | |
| r-value | 0.081 |
| P-value | 0.159 |
| No. | 303 |
The multivariate linear regression analysis of umbilical cord diameter with oligohydramnios and meconium-stained liquor were found significant association than other variants (Table 3).
| Unstandardized Coefficients | t | P-Value | 95.0% Confidence Interval for B | |||
|---|---|---|---|---|---|---|
| B | Std. Error | Lower Bound | Upper Bound | |||
| Oligohydramnios | -0.541 | 0.227 | -2.386 | 0.018 | -0.986 | -0.095 |
| Meconium stained liquor | -1.193 | 0.484 | -2.464 | 0.014 | -2.145 | -0.240 |
Our study showed that 127 newborns were admitted and 176 newborns not required admission (Table 4). Total 15 newborns were expired, all these were associated with antenatal maternal risk factors and less thickness of umbilical cord (Table 4).
| Need Admission | No. | Umbilical Cord Diameter, Mean ± SD | P-Value |
|---|---|---|---|
| Outcome | 0.001 | ||
| Admitted | |||
| Expired | 15 | 9.96700 ± 2.952235 | |
| Discharge | 112 | 9.65420 ± 2.474955 | |
| Not admitted | |||
| Shift | 176 | 10.75896 ± 2.187243 |
5. Discussion
This prospective observational study was conducted to evaluate the association between umbilical cord diameter and maternal antenatal risk factors. This study indicates that umbilical cord diameter is the indictor of health status of newborn. A wide diameter of cord indicates a good health of newborn. Udoh et al., Lee et al. and Tahmasebi and Alighanbari also reported similar results in their study (15-17). The only limitations of these studies were ultrasound guided measurement of cord thickness. Ultrasound measured diameter may have observational bias. Manual measurement of cord thickness is the strength of our study.
In a previously reported study about measurement of umbilical cord thickness after 20 weeks of gestation, they measured thickness by USG guided. They concluded that umbilical cord thickness provide us information about adverse pregnancy outcome (15). We agree with this concept. We measure umbilical cord thickness just after delivery and found newborn with smaller cord thickness need intensive care. Few newborn expired even after intensive care (15).
Newborns with small umbilical cord diameter got admitted for intensive care compare to newborns shifted to mother without need of admission in our study. This is supported by previous study that stat umbilical cord thickness affects the fetal outcome. Umbilical cord thickness can cause low birth weight baby, and adverse pregnancy outcome (18).
Difference in diameter of umbilical cord between male and female neonates was not found significant in this study. This is in contrast with previous studies which all reported thick umbilical cord in males (17, 19-22).
Oligohydramnios is defined as < 5 amniotic fluid index (AFI) (23). Oligohydramnios is the indicator poor outcome of pregnancy and fetal health (24-26). Our study also agreed with this. Eight newborn delivered from mother containing oligohydramnios. All these babies were admitted in intensive care unit.
Many antenatal risk factors were evaluated for its effect on umbilical cord diameter. Oligohydramnios and meconium-stained liquor were found to be associated with the smaller umbilical cord diameter. Our study confirms that umbilical cord thickness strongly associated with meconium stained liquor. Similar result also found in Tahmasebi and Alighanbari study (15).
Comprehensive inclusion of antenatal risk factors and measurement of umbilical cord diameter by digital caliper are strengths of this study while non-inclusion of preterm neonates, not doing serial assessment of fetal umbilical cord diameter by using ultrasonography are some of the limitations.
5.1. Conclusions
Presence of antenatal risk factors leads to thin umbilical cord. This suggests that thin umbilical cord diameter may contribute to the spectrum of placental insufficiency leading to fetal growth restriction and its implication on the neonatal outcome. Thin umbilical cord has been found to be significantly associated with oligohydramnios and meconium-stained liquor. More severe is the oligohydramnios, thinner is the umbilical cord. However no significant correlation was demonstrated between umbilical cord thickness and maternal age and gestation. A large series is needed to confirm these findings and its implication on neonatal outcome.