1. Background
Nephrotic syndrome (NS) is one of the most common glomerular diseases in children (1). The most useful clinical classification in NS relies on response to steroid treatment, including steroid-sensitive nephrotic syndrome (SSNS) and steroid-resistant nephrotic syndrome (SRNS) (2). Both categories have a similar clinical picture on presentation, and till recently, no specific laboratory parameters were detected to distinguish these two clinical entities (3). Noninvasive markers are deeply demanded to predict steroid resistance (SR) in patients with NS before starting treatment, yet there are currently no such markers in practice (4).
The exaggerated excretion of vitamin D binding protein (VDBP) by the kidneys in the form of urinary VDBP (uVDBP) has been demonstrated to be associated with tubular defects (5). The molecular and physical features of VDBP are similar to albumin, molecular weight: 58 vs. 66 kDa, and isoelectric point range: 4.8 - 5.2 vs. 4.8, respectively, and it was recently reported that uVDBP is elevated in patients with NS. Also, uVDBP was found to be higher in patients with SRNS compared to those with SSNS, irrespective of the degree of proteinuria (4). Hence, it was postulated that uVDBP could act as a biomarker for predicting responsiveness to steroid treatment in children with NS.
The age of onset of NS has been demonstrated to influence the disease outcome (6). Some studies have evaluated the predictors of SR by comparing SSNS and SRNS and analyzing total leucocyte count (TLC) (7) and subpopulations of leucocytes (8). Nevertheless, no simple clinical parameters or routine laboratory tests have been introduced as a basic screening tool to inform the clinician of the likelihood of SR before starting steroid therapy.
2. Objectives
In this study, we aimed to study the role of uVDBP as a biomarker of responsiveness to steroid therapy and to identify the presenting clinical-laboratory parameters that could predict the pattern of response to steroids in childhood NS.
3. Methods
3.1. Patients
Sixty patients fulfilling the diagnostic criteria of childhood NS (9) were enrolled in the study upon presenting with active proteinuria; thirty patients were newly presenting (incidental NS), and the other thirty patients had relapsing NS (common NS). Patients were those referring to the Pediatric Nephrology Outpatient Clinic and/or inpatient wards of the Cairo University Children's Hospital (CUCH) over a period of nine months. Patients aged 1 - 12 years and diagnosed with idiopathic NS presenting with the active (proteinuric) disease were included before starting steroid therapy. Patients with impaired kidney function tests, a history of resistance to steroid therapy, and those with congenital or infantile NS were excluded from the study. Thirty age and sex-matched healthy controls were recruited from the General Outpatient Clinic, CUCH, during routine follow-up, as a reference for uVDBP values.
3.2. Methods
3.2.1. Ethical Approval
The study protocol was approved by the scientific ethics committee of the Pediatric Department, Faculty of Medicine, Cairo University (N: I-140417). Informed consent was obtained from patients’ guardians before data collection. All procedures were performed according to the 1964 Helsinki Declaration.
3.2.2. Sample Size Calculation
The sample size was calculated based on a study conducted by Choudhary et al. (10), who measured uVDBP levels in patients with SRNS, SSNS, and healthy controls. The mean values were 701.12, 252.87, and 34.74 ng/mL in the three groups, respectively. With an enrollment ratio of 1, a type I error of 5%, and a study power of 95%, the sample size was calculated as 18 people per group.
3.2.3. Evaluation of Patients with Nephrotic Syndrome Before Starting Steroid Therapy
Demographic characteristics and disease-related data (age of onset, frequency of relapse, and preceding infections) of patients with NS were collected on the day of enrollment. Clinical assessment was performed for all patients. Growth measurements were plotted to the Egyptian Growth charts for age and sex (11), and the percentages of mean values were calculated. Hypertension was defined as systolic BP (SBP) and/or diastolic BP (DBP) ≥ 95th percentile (12). Laboratory data, including complete blood count, kidney function tests (blood urea nitrogen and serum creatinine), serum albumin, total cholesterol, serum electrolytes (Na, K, Ca, and PO4), alkaline phosphatase level, C4 and C4 complement proteins, urine analysis (by dipstick), and urine albumin/creatinine ratio, were measured for all patients with NS on presentation.
3.2.4. Urinary Vitamin D-Binding Protein Measurement
Clean-catch midstream urine samples were collected into sterile plastic containers at the baseline before starting steroid therapy and then centrifuged for five minutes at 5000 rpm to remove all cell debris and particulate matter. The supernatant was stored at -20°C for not more than three months for further analysis. Repeated freeze-thaw cycles were avoided. Urinary vitamin D-binding protein was measured using a Human DBP (vitamin D Binding Protein) ELISA kit (Fine Test, catalog No. EH2937, Wuhan, China). The assay was performed according to the manufacturer’s instructions. A standard curve was drawn using VDBP standard preparations supplied with the assay. Following the colorimetric reaction, optical density (OD) readings at 450 nm were converted into concentrations in ng/mL. The levels of uVDBP were normalized according to urine creatinine (Cr) concentrations and presented as uVDBP: Cr ratio (ng/mg of Cr) (13).
3.2.5. Follow-up of Patients with Nephrotic Syndrome After Steroid Therapy
All patients received full-dose steroid therapy (i.e., a dose of 60 mg/m2/day). The patients were monitored for 4 - 8 weeks after treatment to assess their response to steroids. The endpoint of the study was either achieving remission in response to steroid treatment (SSNS) or failure to respond to eight weeks of treatment with steroids alone (SRNS) (14).
3.3. Statistical Analysis
Data were analyzed using statistical package for the social sciences (SPSS) Software version 25.0 (SPSS Inc., Chicago, IL, USA). Qualitative data were expressed as frequencies and percentages. Quantitative data were expressed as mean and SD if they were normally distributed (parametric). Student t-test was used for two-group comparisons, while the ANOVA test was used for three-group comparisons. For non-normally distributed (non-parametric) quantitative data, median and interquartile ranges were used to present the data, and differences between the study groups were compared by the Mann-Whitney test. The distribution of qualitative data between groups was compared using the chi-square (χ2) test. Receiver operator characteristic (ROC) analysis was used to determine the optimal cut-off values for age, TLC, and platelet count for predicting responsiveness to steroids. Logistic regression was used to identify the independent predictors of steroid resistance. P values < 0.05 were considered statistically significant.
4. Results
4.1. Characteristics of Patients with Nephrotic Syndrome on Presentation
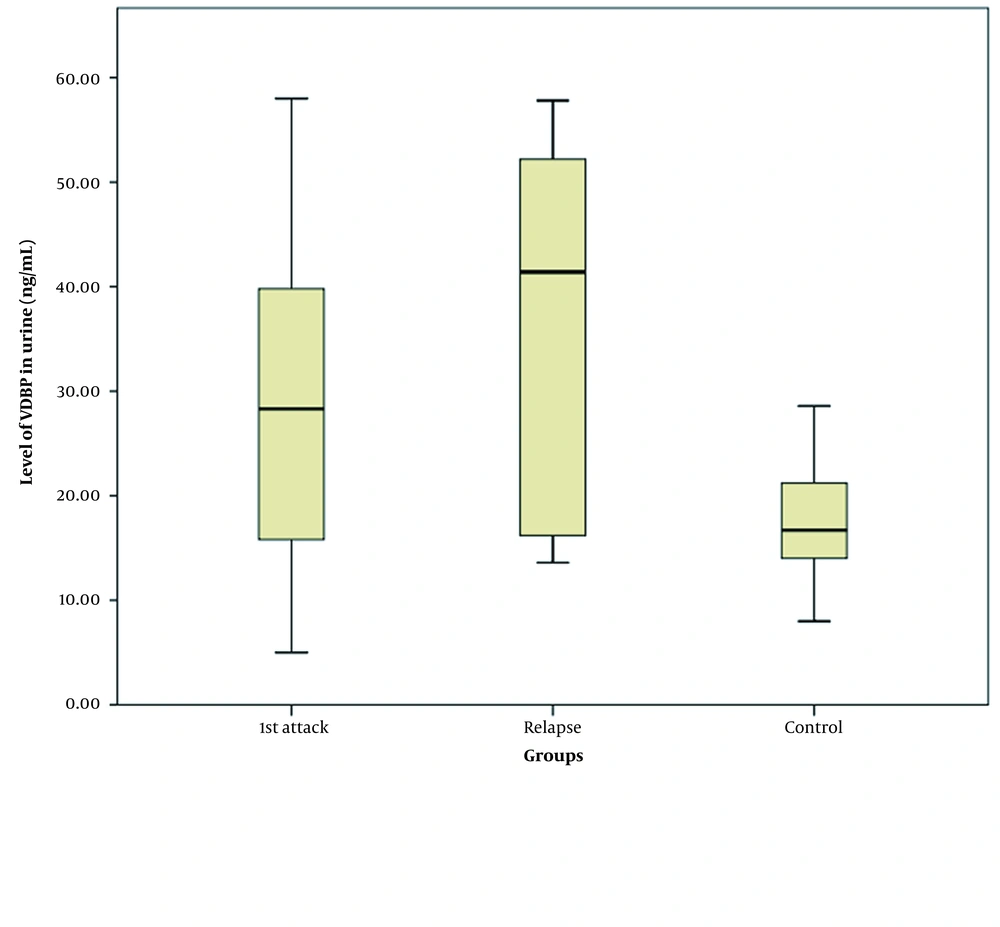
The mean age of patients with NS (n = 60) was 5.43 ± 2.76 years, with a mean age of onset of 4.41 ± 2.6 years and a median duration of illness of 1.5 months (ranging between one week in incidental NS and eight months in common NS). Forty-three patients were males (72%), while 17 were females (28%). Patients in the relapsing NS group tend to show lower BMIs (P = 0.02) and linear growth parameters compared to those presenting with newly diagnosed NS (P = 0.001). Other demographic, clinical, and laboratory data of the patients have been illustrated in Table 1.
| Characteristics | Newly Presented NS (n = 30) | Relapsing NS (n = 30) | P Value |
|---|---|---|---|
| Age (y) | 4.98 ± 2.81 | 5.88 ± 2.69 | 0.15 |
| Age of onset (y) | 4.98 ± 2.81 | 3.85 ± 2.27 | 0.135 |
| Sex | 0.774 | ||
| Male | 22 (73.3) | 21 (70) | |
| Female | 8 (26.7) | 9 (30) | |
| Weight (of mean for age & sex) | 101.58 ± 16.53 | 101.6 ± 15.59 | 0.997 |
| Height (of mean for age & sex) | 98.1 ± 8.78 | 91.08 ± 7.46 | 0.001 |
| BMI (kg/m2) | 22.45 ± 7.68 | 18.54 ± 4.6 | 0.02 |
| Preceding GIT or throat infection | 0.795 | ||
| Yes | 16 (53.3) | 17 (56.7) | |
| No | 14 (46.7) | 13 (43.3) | |
| Blood pressure | 0.573 | ||
| HTN | 8 (26.7) | 10 (33.3) | |
| Normal | 22 (73.3) | 20 (66.7) | |
| Qualitative albuminuria before steroids | 0.492 | ||
| +2 | 0 (0) | 2 (6.7) | |
| +3 | 30 (100) | 28 (93.3) | |
| Qualitative albuminuria after steroids | 0.002 | ||
| 0 | 26 (86.7) | 13 (43.3) | |
| +1 | 0 (0) | 2 (6.7) | |
| +2 | 1 (3.3) | 7 (23.3) | |
| +3 | 3 (10) | 8 (26.7) | |
| uVDBP (ng/mL) | 28.73 ± 13.67 | 37.93 ± 16.2 | 0.014 |
| uVDBP/creatinine (ng/mg) | 31.65 (17 - 76.4) | 52.95 (19.2 - 130) | 0.122 |
| Remission after 4 - 8 weeks | 0.002 | ||
| SSNS | 26 (86.7) | 15 (50) | |
| SRNS | 4 (13.3) | 15 (50) |
Demographic, Clinical, and Laboratory Data of Patients with Nephrotic Syndrome (n = 60) a
4.2. Urinary Vitamin D Binding Protein Levels
Age (P = 0.079) and sex-matched (P = 0.42) healthy controls (n = 30) showed a significantly lower absolute uVDBP mean value (17.35 ± 5.49 ng/mL) than patients with NS (33.33 ± 15.6 ng/mL, P < 0.001). Three-group comparisons between the newly presenting NS, relapsing NS, and control groups showed an elevated absolute level of uVDBP in patients with NS (newly diagnosed: 28.73 ± 13.67 ng/mL, relapsing: 37.93 ± 16.2 ng/mL) compared to control subjects (17.35 ± 5.49 ng/mL, P < 0.001). Post-hoc analysis showed that there was also a more increase in the absolute uVDBP level in patients with relapsing NS (37.93 ± 16.2 ng/mL) compared to those with newly diagnosed NS (28.73 ± 13.67 ng/mL, P = 0.014), as shown in Figure 1. However, after correcting uVDBP levels in relation to urinary creatinine, there was no significant difference in the level of uVDBP/creatinine between patients with newly diagnosed NS (median 31.65, IQR:17 - 76.4 ng/mg) and those with relapsing NS (median 52.95, IQR:19.2 - 130 ng/mg; P = 0.122).
4.3. Steroid Responsiveness
At the end of the follow-up period, 41 patients responded to steroid treatment with complete remission of clinical and laboratory findings (SSNS), while 19 patients were resistant to steroid treatment (SRNS). Patients with newly diagnosed NS had significantly higher rates of remission following steroid treatment than patients with relapsing NS (86.7% versus 50%, P = 0.002).
Table 2 compares baseline characteristics (before starting steroid therapy) between patients with SSNS (n = 41) and SRNS (n = 19). Patients with SSNS had higher age of onset (P = 0.023) and a shorter duration of illness (P = 0.007) than patients with SRNS. At presentation, leukocytosis and thrombocytosis were more pronounced in the SRNS group than in the SSNS group (P = 0.031 and 0.044, respectively).
| Characteristics | SSNS (n = 41) | SRNS (n = 19) | P Value |
|---|---|---|---|
| Age (y) | 5.55 ± 2.52 | 5.18 ± 3.29 | 0.355 |
| Age of onset (y) | 4.87 ± 2.67 | 3.42 ± 2.18 | 0.023 |
| Duration (months) | 6.72 ± 14.7 | 14.88 ± 18.8 | 0.007 |
| Weight (% of mean for age & sex) | 102.63 ± 12.8 | 101.11 ± 17.3 | 0.734 |
| Height (% of mean for age & sex) | 99.65 ± 9.81 | 92.24 ± 7.32 | 0.002 |
| BMI (kg/m2) | 18.57 ± 6.33 | 21.39 ± 6.57 | 0.124 |
| TLC (C/mm3) | 9.08 ± 4.72 | 11.61 ± 4.90 | 0.031 |
| HB (gm/dL) | 11.72 ± 1.94 | 12.27 ± 1.63 | 0.251 |
| PLT (C/mm3) | 472.78 ± 155.25 | 537.21 ± 101.52 | 0.044 |
| Serum Na (mEq/L) | 137.89 ± 5.34 | 137.05 ± 3.85 | 0.488 |
| Serum K (mEq/L) | 4.34 ± 0.65 | 4.49 ± 0.55 | 0.354 |
| Urea (mg/dL) | 24.92 ± 17.43 | 28.10 ± 13.26 | 0.340 |
| Serum Creatinine (mg/dL) | 0.51 ± 0.34 | 0.51 ± 0.20 | 0.411 |
| Serum Calcium (mg/dL) | 7.53 ± 1.00 | 8.02 ± 1.21 | 0.133 |
| Serum Phosphorus (mmol/L) | 5.09 ± 1.01 | 5.17 ± 1.04 | 0.785 |
| Serum ALP (U/L) | 158.37 ± 47.07 | 190.98 ± 152.53 | 0.769 |
| C3 (mg/dL) | 119.26 ± 33.71 | 120.41 ± 31.56 | 0.968 |
| C4 (mg/dL) | 32.63 ± 10.53 | 30.57 ± 13.16 | 0.413 |
| uVDBP (ng/mL) | 34.91 ± 15.01 | 32.60 ± 15.94 | 0.417 |
| uVDBP/creatinine (ng/mg) | 78.26 ± 61.14 | 62.9 ± 81.18 | 0.148 |
Demographic, Clinical, and Laboratory Data of Patients with SSNS and SRNS a
At the end of the follow-up period, there was no significant difference between the SSNS and SRNS groups regarding the absolute uVDBP levels at presentation (34.91 ± 15.01 vs. 32.60 ± 15.94 ng/mL, P = 0.417). Likewise, after correcting baseline uVDBP levels in relation to urinary creatinine, we still found no significant difference in the levels of uVDBP/creatinine between the SSNS and SRNS groups (78.26 ± 61.14 vs. 62.9 ± 81.18, P = 0.148)
4.4. Other Predictors of Steroid Resistance
Multivariate logistic regression analysis was used to determine the predictors of steroid resistance as an outcome. We found that after adjusting for other variables, younger age of onset (OR, 0.733; 95% CI 0.538 - 0.999; P < 0.05) and higher TLC on presentation (OR, 1.155; 95% CI 1.009 - 1.322; P < 0.036) were associated with SRNS.
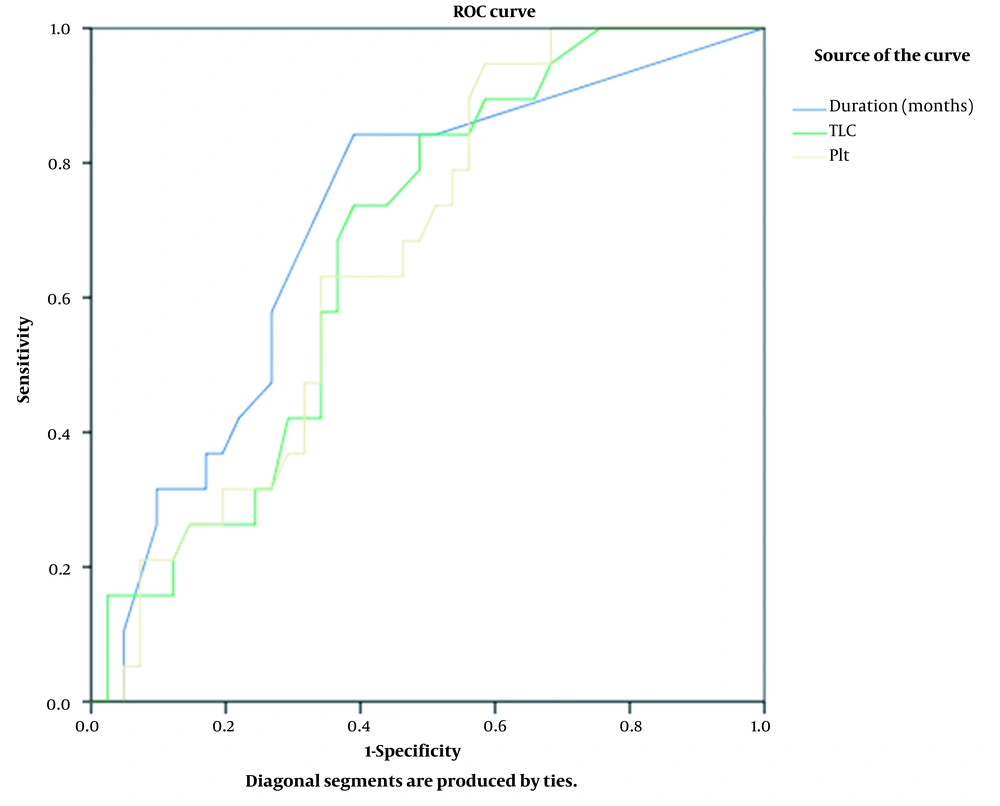
Furthermore, ROC curve analysis was conducted to test the power of TLC on presentation to distinguish patients with SRNS from those with SSNS (Figure 2), showing that the cut-off value of 8.05 × 103/mm3 could distinguish SRNS from SSNS with a sensitivity of 84% and specificity of 51%. The area under the curve (AUC) was 0.675 (95% CI 0.539 - 0.81, P = 0.031), indicating an acceptable predictive power.
Regarding platelet count on presentation, the cut-off value of > 516.5 × 103/mm3 was able to distinguish SRNS, as an outcome, from SSNS with a sensitivity of 63% and specificity of 66%. The AUC was 0.66 (95% CI 0.526 - 0.799, P = 0.04), reflecting an acceptable predictive power.
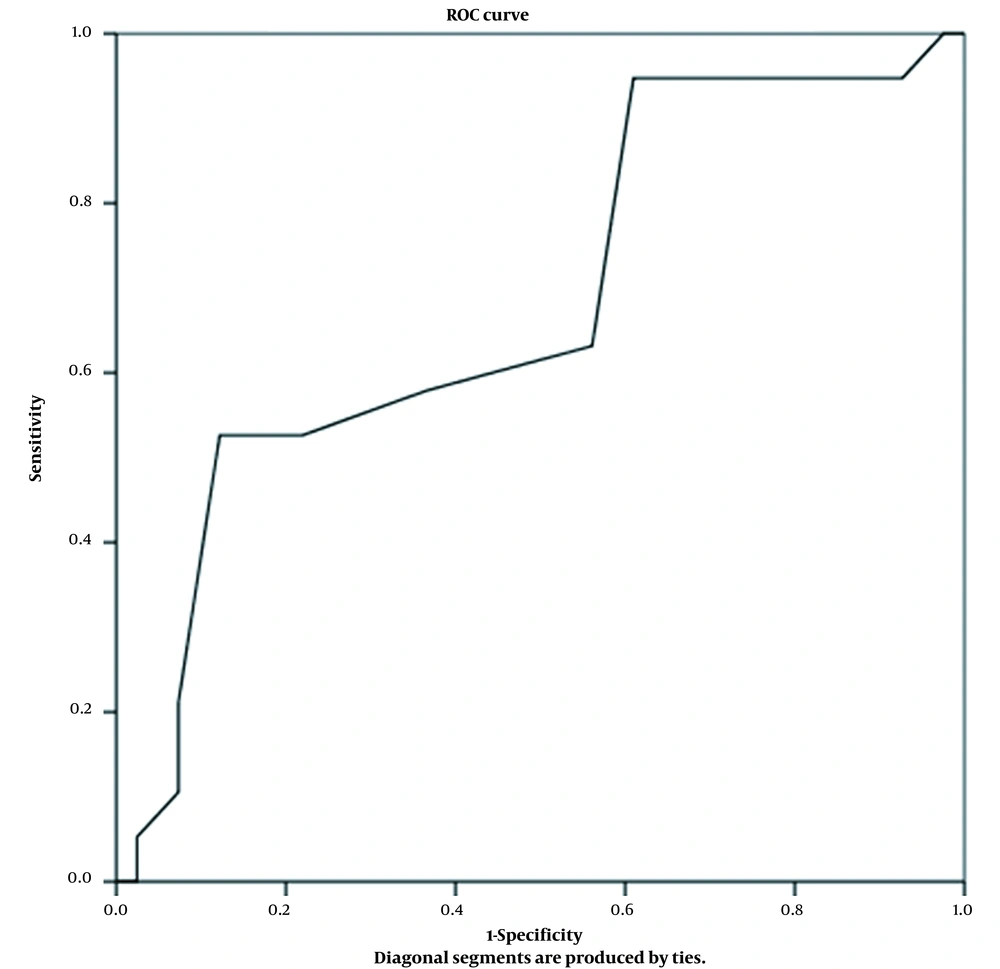
Regarding the age of onset as a predictor of steroid responsiveness, ROC curve analysis showed that at a cut-off value of fewer than 2.1 years, SR could be predicted with a specificity of 88% and sensitivity of 53%. The AUC was 0.68 (95% CI 0.534 - 0.832, P = 0.02), reflecting an acceptable predictive power (Figure 3).
5. Discussion
Despite research advances, there is no early noninvasive clinically applicable predictor of steroid responsiveness in childhood NS (15). So far, the patient’s clinical response to steroids and renal histopathology are the foremost guidelines for clinicians (16). Here, in a longitudinal analysis, we assessed the association between the presenting features of childhood NS and the pattern of response to 4 - 8 weeks of steroid therapy. We also tested the potential predictive value of uVDBP levels, as an understudied factor, for steroid responsiveness. The results of this study revealed that there were no significant differences in the baseline uVDBP levels (P = 0.417) or uVDBP/creatinine ratio (P = 0.148) between patients who had SSNS or SRNS after 4 - 8 weeks of steroid therapy. In our study, we demonstrated that SR was significantly associated with younger age of onset (P = 0.023), longer duration of illness (P = 0.007), relapsing disease (P = 0.002), and elevated total leukocyte (P = 0.031) and platelet (P = 0.044) counts on presentation. We identified that the best cut-off values that could differentiate between SSNS and SRNS were an age of onset of < 2.1 years, TLC at diagnosis > 8.05 × 103/mm3, and platelet count on presentation > 516.5 × 103/mm3.
The results of this study revealed that uVDBP absolute levels at the time of diagnosis were significantly higher in patients with NS compared to healthy controls (P < 0.001). Also, those with relapsing NS had significantly higher uVDBP absolute levels compared to those experiencing the first attack of NS (P = 0.014). However, there was no significant difference in the baseline uVDBP/creatinine ratio (P = 0.148) between the SSNS and SRNS groups. Many previous studies investigating VDBP in NS patients have already reported a strong positive correlation between uVDBP and proteinuria (4, 17, 18). Our findings also outlined that uVDBP could reflect disease severity (in terms of both proteinuria and tubulointerstitial damage) rather than directly predict steroid responsiveness. It was hypothesized that damaged tubular epithelial cells might no longer be capable of scavenging VDBP, resulting in its gross loss via urine (19), and not only as a part of proteinuria in NS. This hypothesis was supported by the findings of Chaykovska et al. (20), noting an increase in uVDBP in parallel with the severity of renal damage and independently of albuminuria in a rat model.
Choudhary et al. investigated the association between uVDBP levels and steroid responsiveness in children with idiopathic NS and found that uVDBP levels were significantly higher in patients with SRNS than in patients with SSNS (701.12 ± 371.64 vs. 252.87 ± 66.34 ng/mL, P < 0.001) (10). Bennett et al. conducted a cross-sectional study on children with SRNS (n = 24), SSNS (n = 28), and healthy controls (n = 5) and suggested that uVDBP could represent a noninvasive biomarker to distinguish SRNS from SSNS (4). The results were very promising; nevertheless, both studies had some limitations. As mentioned by Aoun in his review (21), the cross-sectional nature of both studies and the timing of urine sample collection to check VDBP levels (patients were already on steroid treatment without checking uVDBP levels at the baseline) could have profoundly affected the results, and changes in uVDBP levels after treatment could be attributed to the remission of proteinuria rather than being a predictor of steroid responsiveness. This means that with remission of proteinuria, uVDBP excretion decreases, explaining why uVDBP levels were lower in the SSNS group than in the SRNS group; however, this biomarker was not a valid indicator of steroid responsiveness.
We designed this cohort study to overcome the drawbacks of prior cross-sectional studies. The urine samples for measuring VDBP levels were collected from patients at the baseline, and the data were analyzed 4 - 8 weeks after steroid therapy. Baseline uVDBP levels were then compared between patients with SSNS and SRNS to evaluate the validity of uVDBP as a biomarker of steroid responsiveness. We could not demonstrate any significant difference in the absolute levels of uVDBP at presentation between children with SSNS or SRNS (P = 0.417) at the end of the follow-up period, even after adjustment for urinary creatinine (P = 0.148). In our study, we found that uVDBP levels were higher in patients with relapsing NS than those with newly diagnosed NS. This is in agreement with the observation that uVDBP reflects the disease severity but cannot predict steroid responsiveness. Thus, our results do not rule out uVDBP as a good potential biomarker to monitor tubule-interstitial damage in NS; however, its relationship with histological features and clinical outcomes has to be further validated.
The age of onset of NS is an important factor warranting further management of relatively young (< 1 year) and old patients (> 10 years) who do not show a promising response to steroid therapy (6). In a report published by Nourbakhsh and Mak, they documented that one of the key features in patients with NS was the age below six years (especially younger than two years) at the time of diagnosis, suggesting genetic testing for diagnosis (22). Moreover, Benoit et al. suggested a systematic approach for genetic testing based on the age of diagnosis, histopathologic findings, and mode of inheritance. They included patients who were aged from three months to two years at the time of NS presentation (i.e., infantile NS) (23), whose response to steroid therapy was different from childhood NS. Today’s guidelines for the management of NS suggest the initiation of steroid therapy for NS diagnosed beyond one year of age. However, in our study, we demonstrated that a cut-off value of < 2.1 years predicted a tendency toward developing the SRNS phenotype. So, clinicians are recommended to consider histopathological analysis and genetic testing for this age group and, whenever possible, avoid using steroids for long periods in children with NS.
Similar to our results, it was reported that patients with relapsing NS had significantly higher TLC than those newly diagnosed with NS, which is likely due to the fact that most NS patients experience infections at the time of relapse (7). Also, Dakshayani et al. reported concomitant infections (represented by elevated TLC) as a significant risk factor for frequent relapses, reflecting a poor response to steroid therapy (24). In the present study, almost half of patients with NS had a preceding infection to the onset of NS; however, leucocyte differential counts were unavailable, which could be a limitation of our study concerning the role of TLC in the prediction of SR. Nevertheless, elevated TLC can be used as a prognostic factor for both risks of relapse and SR.
Thrombocytosis has been previously reported in children with NS (25, 26). Platelet count was reported to be high during remission and early after the cessation of treatment, starting to become normalized during long-term remissions (25). Contradicting these reports, Mittal et al. described normal PLT counts in Indian children with NS (27). To the best of our knowledge, there are no previous reports on the correlation between PLT count on presentation and the pattern of steroid responsiveness.
To date, our results represent the only available data based on a prospective longitudinal study where uVDBP levels were checked using urine samples collected from patients with NS at the baseline and before starting steroid therapy, which is a major strength of our study. However, our study has some limitations. It was a single-center study among a cohort of Egyptian patients with NS without involving other racial/ethnic populations. Moreover, the small number of patients in our cohort could be another limitation worth mentioning. Further, the sensitivity and specificity of some predictors of SR are only modest. Finally, leucocyte differential counts on presentation were unavailable, which could be another limitation of our findings concerning the role of TLC in predicting SR.
It is recommended to conduct a multicenter prospective study involving more children with idiopathic NS with longer periods of follow-up. We also recommend that any future study should take into consideration that uVDBP levels should be checked at the baseline and before initiating steroid therapy to abolish the potential confounding effects of steroids. Although uVDBP was not a valid biomarker of steroid responsiveness in children with NS enrolled in our cohort, its relation to disease severity was highlighted as uVDBP levels were higher in relapsing NS than in newly diagnosed NS, needing further exploration.
5.1. Conclusions
There was no significant difference in the absolute uVDBP level at the baseline between children with SSNS or SRNS after 4 - 8 weeks of steroid therapy, even after adjusting for urinary creatinine. Urinary VDBP was higher in patients with NS compared with healthy counterparts, with a more pronounced increase in relapsing than newly diagnosed NS, suggesting a role for this biomarker as an indicator of disease severity rather than the pattern of steroid responsiveness. Younger age of onset, longer disease duration, previous relapses, and increased TLC and PLT count on presentation were associated with SR in childhood NS. Age of onset (< 2.1 years) and TLC (> 8.05 × 103/mm3) were independent predictors of SR. Therefore, these parameters can serve as red flags that alarm the clinician not to extend the administration of steroids without further interventions in limited-resource settings.