1. Background
Neonatal sepsis is a major cause of morbidity and mortality. Of the 6.9 million neonatal sepsis burden, South Asia accounts for 3.5 million cases per year. India, with its 1.6 billion population, claims a large proportion of this disease burden (1). Neonatal sepsis is defined, by the 2002 International Pediatric Sepsis Consensus Conference, as systemic inflammatory response syndrome in the presence of or as a result of suspected or proven infection. But so far, a worldwide agreement on the definition of early-onset neonatal sepsis (EOS) has not been reached. It encompasses various systemic infections of the newborn such as Septicemia, Meningitis, Pneumonia, Arthritis, Osteomyelitis, and Urinary Tract Infections. A number of bacterial and non-bacterial agents may infect newborn in utero, intra partum, or post-partum. Intrauterine transplacental infection is significant to the fetus and/or newborn including syphilis, rubella, CMV, ParvoB19, and varicella. Although HSV, HIV, Hep B, Hep C, and TB can result in transplacental infection, the most common mode of transmission for these agents is intra-partum, during labor and delivery with passage through an infected birth canal (HIV, HSV, Hep B virus), or postpartum, from contact with an infected mother or care taker (2).
Bacterial sepsis is the main cause of death in infants, and one of the three primary causes of 2.7 million deaths every year. Early onset sepsis (EOS) refers to sepsis in neonates at or before 72 hours of life, and late onset sepsis (LOS) is defined as sepsis occurring at or after 72 hours of life. Various sources can be the origin of sepsis, one of which is the infected umbilical cord that can lead to cellulitis and eventually sepsis; fungal and viral infections can occur in the setting of prematurity through vertical transmission from the mother (3). Neonatal Viral infections are common yet under-recognized. Viral infections are less often suspected and diagnosed than bacterial infections in neonates (4). There is scarcity of literature and only a few studies have explored the viral neonatal infections (5-8).
Viral studies are not routinely conducted on neonates due to the overlapping clinical signs and symptoms, the difficulty in obtaining laboratory confirmation of a viral infection, and the lack of effective therapeutic interventions. Recent developments in rapid and highly sensitive diagnostic methods, such as PCR, have greatly improved diagnosis of virus infections.
2. Objectives
This study aimed to find the incidence and proportionate distribution of neonatal viral infection in newborns admitted to NICUs.
3. Methods
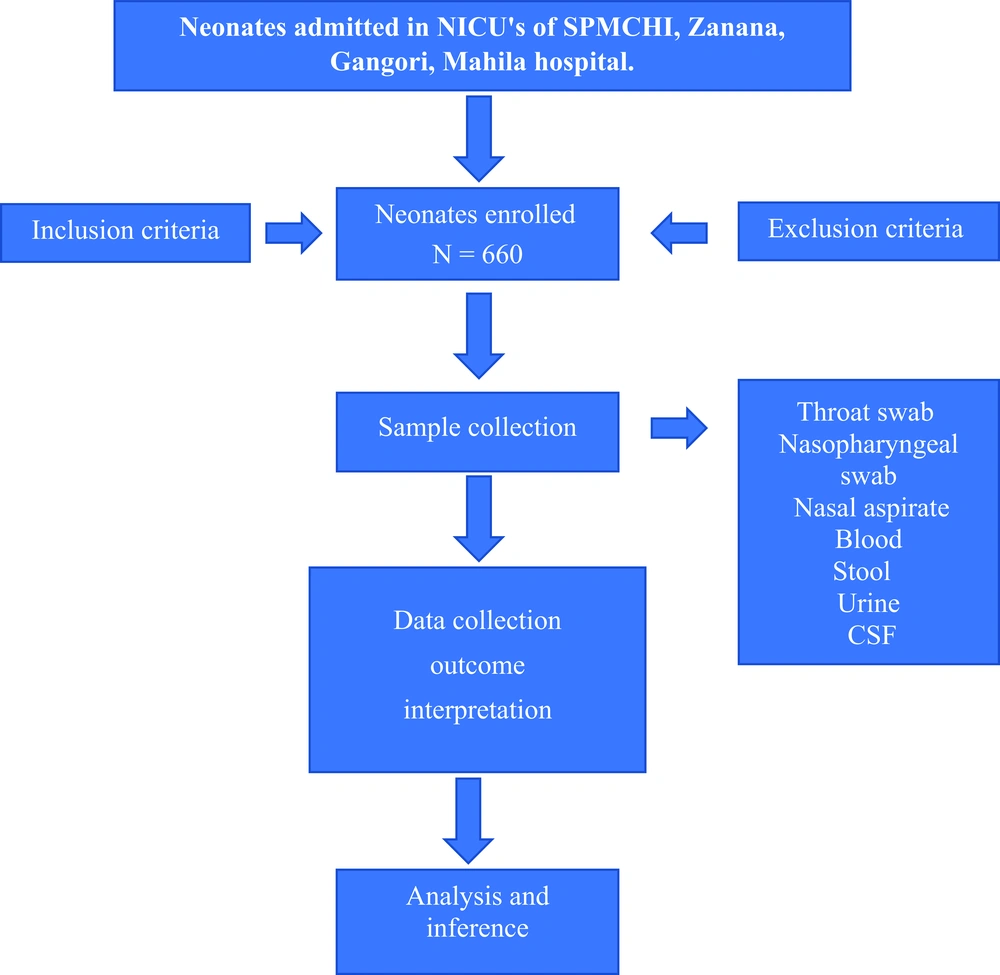
This observational, cross-sectional, descriptive study was conducted from June 2019 to 15 October 2020 in NICUs affiliated to the department of pediatrics at SPMCHI, Zanana hospital, Mahila hospital, Gangotri hospital, and S.M.S. medical college in Jaipur Rajasthan after obtaining clearance from research review board of the institute. The sample size was calculated at a/an confidence level of 95%, alpha error of 5%, assuming viral infection positively 1.7% among newborns admitted in NICUs, and absolute allowable error of 1%. Then the estimated sample size became 660 newborns. Categorical/Nominal variables were expressed as numbers and percentages and were analyzed using the Chi-square test. All statistical analysis was performed using SPSS trial version 20.
All newborns admitted in NICUs for suspected clinical sepsis were included in this study except for newborns with congenital malformation, HIE, PT for care, and negative consent. Therefore, a total of 660 subjects of suspected sepsis were divided into two groups based on microbiological tests positive for viral infection: (Figure 1: Flow chart)
(1) Group one included 560 newborns who were non-virus sepsis.
(2) Group two included 100 newborns who were positive for virus infection according to microbiological tests (virus sepsis).
Sampling procedures for routine and advanced diagnostic virology tests: First, 2 mL of blood samples was collected in EDTA vial for CBC, and 2 mL blood samples was collected for C-reactive protein (CRP), viral markers for meningitis, HIV, Hep B, toxoplasmosis, other, rubella, cytomegalovirus, herpes simplex virus (TORCH) group of infections in the plane vial. Stool samples were screened for Rotavirus and Adenovirus. Urine samples were sent for CMV infection, and nasopharyngeal and throat swabs were sent for RSV, Metapneumovirus, Para influenza virus, H1N1 virus, Adenovirus, and Influenza virus. Then 1 mL of CSF sample was sent from suspected newborns with meningitis for Herpes virus, CMV, and Parvovirus (Table 1). All samples were sent with the maintenance of cold chain temperature between 40°C to 80°C to Advance Virology Lab., Department of Microbiology, SMS Medical College Jaipur.
| Clinical Presentation | Kind of Sample | Virus Isolated |
|---|---|---|
| 1. Bronchiolitis | Nasopharyngeal swab or nasal aspirate or throat swab | RSV, Metapneumovirus, Para influenza, H1N1 virus, Adenovirus, Influenza virus |
| 2. Abdomen distension, diarrhea, GI symptoms | Stool | Rota virus, Adeno virus |
| 3. Seizure or encephalitis | CSF, Urine | Herpes, CMV |
| 4. Sepsis like illness | Blood | Enterovirus, Parvovirus, Ebstein-Barr virus. |
| 5. History of fever with rash in mother | CSF | Varicella zoster virus |
| 6. Hepatosplenomegaly, cholestasis, chorioretinitis, Microcephaly, cataract, micropthalmia | Blood | TORCH group |
| 7. Microcephaly, cutis gyrata, fetal brain cleft or hydrocephalus | Blood, Urine | Zika virus |
| 8. Mother HBsAg positive with hepatitis | Blood | Hepatitis B virus |
| 9. Maternal HIV infection present | Blood | HIV |
| 10. Localised skin, eye, mouth or mucocutaneous membrane lesion, Seizure, shock, respiratory distress and DIC | Stool, Urine & CSF | Herpes simplex virus |
Reverse transcriptase- polymerase chain reaction (RT-PCR) is used to qualitatively detect gene expression through creation of complementary DNA (cDNA) transcripts from RNA. This technique is commonly adopted in molecular biology to detect RNA expression. This technique was implemented in our institute (i.e., microbiology department) in 2009. To this end, 7500 Fast Dx RT-PCR was used, which took 90 min. Various specimens were obtained from different sources to isolate organism like stool, throat swab, CSF, Blood, etc. PCR was used to detect viral DNA having sensitivity of 75% to 100% and specificity of 71% to 100%.
Enzyme linked immuno-sorbent assay (ELISA) is a quantitative immunological procedure in which the Ag-Ab reaction is monitored by enzyme measurements. It is a plate-based assay technique designed for detecting and quantifying substances such as peptides, proteins, antibodies, and hormones. DIA PRO kit was used at microbiology department, SMS Medical College. Time-take period for ELISA is 2 h. Diagnostic sensitivity and specificity of ELISA for HSV IgM, CMV IgM, and Rubella IgM are > 98%. The technique is commonly used in molecular biology to detect microbes in various specimens from different sources and to isolate organism like stool, throat swab, CSF, Blood etc.; the tests based on detection of RNA expression (RT-PCR) or quantitative immunological procedure (ELISA) are considered as microbiological tests.
4. Results
Out of 660 subjects, 490 (74.24%) were males and 170 (25.76%) were females; 572 (86.70%) were Hindus and 88 (13.3%) were Muslims. Out of 660, 420 were full term and 240 were pre-term, 609 (92.3%) were appropriate for gestational age (AGA), 50 (7.5%) were small for gestational age (SGA), and 1 (0.2%) was large for gestational age (LGA). Out of 609 AGA neonates, 539 (88.5%) had non-viral sepsis and 70 (11.5%) had viral sepsis. Out of 50 SGA neonates, 21 (42%) had non-viral sepsis and 29 (58%) had viral sepsis. One LGA neonate was diagnosed with viral sepsis. Distribution of the subjects among two groups was statistically significant according to birth weight in relation to gestational maturity (P-value = 0.001) (Table 2).
| Parameters | Group One (n- 560) | Group Two (n-100) | P-Value |
|---|---|---|---|
| Gender | - | ||
| Male | 411 | 79 | |
| Female | 149 | 21 | |
| Age of onset | 0.082 | ||
| Early onset sepsis | 319 | 47 | |
| Late onset sepsis | 241 | 53 | |
| Hemoglobin (gm/dL), mean ± SD | 15.43 ± 3.64 | 14.60 ± 3.93 | 0.038 |
| Total leucocyte count (per mm3), mean ± SD | 13728 ± 12172 | 13601 ± 8004 | 0.920 |
| Platelets counts (per mm3) (mean ± SD) | 225062 ± 13957.10 | 199297 ± 126379.60 | 0.001 |
| Mean transaminase level (IU/L), mean ± SD | |||
| SGOT | 78.62 ± 101.62 | 115.57 ± 183.10 | 0.004 |
| SGPT | 115.57 ± 183.10 | 91.15 ± 167.57 | 0.001 |
| CRP level | 0.325 | ||
| Positive | 425 | 81 | |
| Negative | 135 | 19 |
Distribution of the subjects according to gestational maturity in two groups suggested that out of a total of 660 neonates, 420 (63.6%) were full-term and 240 (36.4%) were pre-term. Out of 420 full-term babies, 333/420 (79.3%) were diagnosed with non-viral sepsis and 87 (20.7%) were diagnosed with viral sepsis. Out of 240 pre-term neonates, 227 (94.6%) had non-viral sepsis and 13 (5.4%) had viral sepsis.
Early onset sepsis (EOS) was found in 366 (55.5%) subjects, and late onset sepsis (LOS) was determined for 294 (44.5%) subjects. No significant difference was observed among newborns with viral sepsis and non-viral sepsis. Out of 100 positive subjects, the most common symptom was petechiae/purpura (11%), and the most common sign was hepatosplenomegaly (42%). The mean hemoglobin (Hb) level of the subjects in group one (i.e., non-viral) was 15.43 ± 3.64, whereas the mean Hb level of the subjects in group two (i.e., viral) was 14.60 ± 3.93; and the difference was found to be significant. The mean total leukocyte count (TLC) of the subjects in group one was 13728 ± 12172, and the mean TLC of the subjects in group two was 13601 ± 8004; and the difference was found to be non-significant. Newborns with viral sepsis had highly significant lower mean platelet counts in comparison to newborns with non-viral sepsis. The mean transaminase level observed in our study was extremely higher in newborns with viral sepsis in comparison to newborns with non-viral sepsis. P-value was highly significant (P-value - 0.001). Out of our 660 subjects, 506 subjects were found positive based on CRP (76.70%) (Table 2).
Viral infection was diagnosed in 100 (15.15%) subjects out of 660 studied subjects who were admitted to NICUs. CMV infection (9.1%) was found to be the most common cause of viral sepsis followed by Rubella (4.5%), HSV (3.8%), H1N1 (1.2%), and Rotavirus (1.1%). Other detected viruses were Epstein-Barr virus (EBV) (0.8%), RSV (0.8%), VZV (0.5%), hepatitis-B (0.3%), enterovirus (0.3%), metapneumo virus (0.2%), rhinovirus (0.2%), Paraechovirus (0.2%), and human immunodeficiency virus (HIV) (0.2%) (Table 3).
| Name of Virus | Number of Positive Tests | % of Positive Test Out of Total Subject (660) |
|---|---|---|
| Cytomegalovirus | 60 | 9.10 |
| Rubella | 30 | 4.50 |
| Herpes simplex virus | 25 | 3.80 |
| H1N1 virus | 08 | 1.20 |
| Rotavirus | 07 | 1.10 |
| Epstein-Bar virus | 05 | 0.80 |
| Respiratory syncytial virus | 05 | 0.80 |
| Varicella zoster virus | 03 | 0.50 |
| Hepatitis-B virus | 02 | 0.30 |
| Enterovirus | 02 | 0.30 |
| Metapneumo virus | 01 | 0.20 |
| Rhinovirus | 01 | 0.20 |
| Paraechovirus | 01 | 0.20 |
| Toxoplasma | 01 | 0.20 |
| Human immunodeficiency virus | 01 | 0.20 |
| Total | 152 | 23.40 |
As for the samples from our 660 subjects, blood samples from 437 (66.20%) subjects, CSF samples from 122 (18.5%) subjects, throat swab samples from 63 (9.54%), stool samples from 24 (3.6%), and urine samples from 14 (2.1%) subjects were analyzed (Table 4).
| Type of Sample | Over all Negative Samples (%) | Sample Specific Negative Samples (%) | Over All Positive Samples (%) | Sample Specific Positive Samples (%) | Total Number of Samples (%) |
|---|---|---|---|---|---|
| Blood | 374 (66.80) | 85.60 | 63 (63) | 14.40 | 437 (66.20) |
| CSF | 118 (21.10) | 96.70 | 04 (04) | 3.30 | 122 (18.50) |
| Throat swab | 049 (08.70) | 77.80 | 14 (14) | 22.20 | 63 (9.54) |
| Stool | 016 (02.90) | 66.66 | 08 (08) | 33.40 | 24 (3.60) |
| Urine | 003 (00.50) | 21.40 | 11 (11) | 78.60 | 14 (2.10) |
| Total (%) | 560 (100) | 84.85 | 100(100) | 15.15 | 660 (100) |
The total number of microbiological positive (RT-PCR, ELISA) subjects afflicted with viral infection was 100, while the total number of the microbiological positive test for viral infection was 152. Out of 660 study subjects, 100 (15.15%) subjects were confirmed to have microbiological viral sepsis. Out of a total of 60 microbiological positive test subjects for CMV, isolated CMV was present in 29 subjects (48.33%), and CMV along with other viruses was present in 31 (51.67%) subjects. Out of a total of 30 microbiological positive test subjects for rubella, isolated rubella was present in 2 subjects (6.67%), while rubella along with other viruses was present in 28 (93.33%) subjects. Out of a total of 25 microbiological positive test subjects for HSV, isolated HSV was present in 7 subjects (28%), and HSV along with other viruses was present in 18 (72%) subjects. Out of a total of 8 microbiological positive test subjects for H1N1, isolated H1N1 was present in 7 subjects (87.5%), and H1N1 along with other viruses was present in 1 (12.5%) subject. Out of a total of 5 microbiological positive test subjects for EBV, isolated EBV was present in 3 subjects (60%), and EBV along with other viruses was present in 2 (40%) subjects.
5. Discussion
Majority of the subjects in our study were male (Table 1) since male newborns outnumbered female newborns in our country. Similar results were observed by Picone et al. (9) and Santos et al. (10).
Out of 100 viral sepsis group subjects in our study, 47% and 53% of the subjects were found to have EOS and LOS, respectively, but no statistically significant difference was detected between the two groups (Table 1). Similar results were observed in the study by Cicirello et al (11), in which 47.3% and 52.7% of the infants in the viral sepsis group were discovered to have EOS and LOS, respectively. Taking into account our study results and the findings from the given study, it was suggested that viral sepsis was more common in subjects of LOS.
As to the symptom and sign for 100 positive subjects, the most common symptom was petechiae/purpura (11%) and the most common sign was hepatosplenomegaly (42%). A statistically significant difference was detected between group one (non-viral sepsis) and group two (viral sepsis) in terms of the mean hemoglobin levels (Table 1). Viral sepsis can bring down the hemoglobin level through various mechanisms. The first mechanism is the increased hemolysis, the second one is an increasing tendency for early hemorrhage, and the third one is the suppression of bone marrow erythropoiesis in severe viral sepsis. Although three mechanisms are also involved in non-viral sepsis, the magnitude is higher in viral sepsis. The above fact explains the lower hemoglobin level in the viral sepsis group as compared to the non-viral sepsis group. Andrea Ronchi et al. (12) observed a median Hb level of 10.3 (9.4 - 12.8) in virus sepsis infants and a median Hb level of 11.8 (10.1 - 13.7) in the non-viral sepsis group. Syriopoulou et al. (13) reported a mean hemoglobin level of 13.2 (9.6 - 16.2) in viral sepsis infants.
The mean TLC value was significantly lower in the viral sepsis group than that in the non-viral sepsis group, but the difference was not statistically significant. The commonly described finding in viral sepsis, suggesting either the normality of TLC or its slightly lower count, was not substantiated based on our observation in this study. Syriopoulou et al. (13) observed a mean total leucocyte count of 9200 (5100 - 19 500) in the viral sepsis group. Ronchi et al. (12) also reported a mean TLC count (per mm3) of 9785 (5600 - 12250) in virally infected newborns as well as a mean TLC count of 11535 (8740 - 16288) in non-viral infected newborns.
In our study, subjects with viral sepsis had significantly lower mean platelet counts in comparison to newborns with non-viral sepsis (Table 1). Syriopoulou et al. (13) found that mean total platelet counts was 223 (121 - 333) × 103 in viral sepsis infants. Pinninti et al. (14) discovered thrombocytopenia (less than 1lkahs per mm3) in 77% of infants.
In our study, subjects from group two had significantly higher mean transaminase (SGOT and SGPT) levels in comparison to newborns from group one (Table 1). Furthermore, the mean transaminase level was found to be higher in the viral sepsis group than that in the non-viral sepsis group. In case this observation was supported by further studies, then SGOT and SGPT levels may have served as an important biochemical marker of viral sepsis. Contrary to our finding, the result from the study by Syriopoulou et al. (13) suggested the mean (SGOT) level of 35 (15 - 66) and mean SGPT level of 26 (6 - 52) in viral sepsis newborns.
The most extensively used and investigated acute phase reactant is CRP. In our study, the CRP > 6 gm/dL was employed as a cut-off point for defining sepsis. CRP was found to be elevated in non-viral sepsis and viral sepsis group, but the difference was not statistically significant (Table 1). Davis et al. (15) indicated that CRP values were not elevated in viral sepsis newborns, but they were elevated in non-viral sepsis group in 4 (5.4%) newborns.
Out of 660 subjects, 100 (15.15%) ones were confirmed to be afflicted with microbiological viral sepsis. CMV infection (9.1%) was determined to be the most common cause of viral sepsis followed by Rubella (4.5%), HSV (3.8%), H1N1 (1.2%), and Rotavirus (1.2%) (Table 2). In developed countries, CMV transmission occurs in 0.5% - 2% of all live births, making CMV infection the most common congenital viral infection (15, 16). In the study by Verboon-Maciolek et al. (17), viral infection was confirmed in 51 (1%) newborns admitted to the NICU. In the given study, it was also found that the enterovirus and parechovirus (EV/PEV) infections were most common (39%) in their study group followed by respiratory syncytial virus (RSV) infection (29%) and rotavirus infection (10%). Multiple test positivity for viruses can be attributed to the poor socio-economic status and overcrowding seen in India due to high population load. Hence, the high overall incidence of the mixed infection in our study may have been explained. Studies conducted in western world have recorded a decreased incidence of multiple viral infection due to generally lesser population, better hygiene, and higher education level of mothers.
In the study by Águeda et al. (18), viral infection was diagnosed in 1.7% (n = 68) of the infants admitted to the NICU. In the given study, the most common viral infection was determined to be respiratory syncytial virus (32.3%), the second most common was detected to be metapneumovirus (17.9%) and influenza H1N1 (17.9%) followed by cytomegalovirus (13.4%). The incidence of TORCH group infection in our study was 10.45%, which was in agreement with the study result of Deorari et al. (19). They carried out a prospective study on the incidence of intrauterine infection by screening 1302 blood samples, out of which 270 (20.6%) were positive for TORCH infection. This finding may have been also attributed to the clinical condition of the newborn since most of them were affected by fetal and maternal disease after referring to the NICU, and since the study population was Indian.
Civardi et al. (20) revealed that 64 (10.8%) newborns out of 590 ones were positive for viral infection. The proportional distribution of a positive virus, Rotavirus, in 64 newborns was found to be 23.44%, and Rotavirus was determined as the most common virus in the studied area, followed by RSV (17.19%) and enterovirus infections (15.63). Santos et al. (10) found CMV infection in 20 (6.8%) newborns out of 292 ones. In our study, higher rate of virus isolation was detected in the samples compared to the rate documented in above studies because the variations of test positivity in different studies were different and our laboratory was an accredited and designated national laboratory. Technology has considerably evolved and techniques for diagnosing the viruses have become more sophisticated in last decade. Viral transport media advancement has improved positivity.
The TORCH titers are widely used for diagnosing neonatal viral infections. Limitations of TORCH screening in IUGR and SGA neonates have been the subject of a few studies (21-23). Routine Screening has been determined to have low utility and high expenses owing to low incidence in general population. Only three out of 23 SGA neonates in a Canadian study (21), nine out of 117 SGA neonates in a USA study (22), and one out of 75 SGA in another USA study (23) were found positive. In our study, the cases with TORCH positive alone were excluded and the result did not percolate on diagnostic virology-based tests. The finding of IgG antibody suggests that preconception maternal immunity is neither diagnostic nor protective. Newer polymerase chain reaction (PCR) based on amplification of viral nucleic acids is highly sensitive to rapid pathogen-specific diagnosis and, therefore, diagnostic virology is more appropriate than serology. In our study, a high rate of viral infection detection was observed, which may have been due to the high sensitivity of the PCR technique; the higher prevalence may have been also attributed to the high prevalence of CMV infection in the Indian population. The high prevalence in the Indian population may have been dealt with in the future once CMV vaccination became available, which was under trial phase at the time of our study.
Many viral diseases in newborns are undiagnosed or subjected to late diagnosis. Therefore, it was recommended that the clinicians should consider specific risk factors instead of classical consideration of TORCH syndromes, since specific signs, symptoms, and findings are different depending on the pathogen under consideration. The availability of Antiviral therapies signifies the importance of specific diagnosis. Laboratory studies of virologic detection (molecular assays) are more sensitive than serologic diagnosis. In addition to pregnant women screening for TORCH infection, the examination of newborns in advanced virology labs with accreditation is needed for establishing the early diagnosis of, at least, commonly prevalent neonatal viral infections.
Our study was a single center study; therefore, our findings may have been generalizable only after performing further studies on the given issue. Our study was carried out at a tertiary care center where facilities for advance virology labs with accreditation to diagnose commonly prevalent neonatal viral infections were available.
5.1. Conclusions
Viral infections accounted for a significant proportion of neonatal sepsis. Therefore, it was recommended that viral infections should be considered in the differential diagnosis of newborns with clinical features, laboratory abnormalities, or signs of neonatal sepsis. It was also suggested that all neonatal units should perform evaluations aiming at diagnosing viral infections. A high TORCH infection positivity rate was observed in this study; therefore, it was recommended that all pregnant women should be screened for TORCH infection, and all neonatologists and pediatricians involved in neonatal care should both suspect a viral agent as a possible cause of sepsis and utilize the diagnostic and treatment facilities with antiviral agents whenever and wherever possible. It was found absolutely necessary to conduct further studies on not only the field of viral isolation but also on the field of development of newer and specifically effective antiviral drugs.