1. Introduction
Recurrent fever is defined by three or more febrile episodes (> 38°C) of fever in a 6-month period occurring at least seven days apart (1). If unexplained bouts of recurrent fever occur with a characteristic frequency and constellation of the symptoms, then a diagnosis of periodic fever syndrome can be considered after ruling out infections due to immunodeficiency and organ malformation (2).
Periodic fever syndrome (PFS) comprises a clinically distinct set of monogenic autoinflammatory disorders that occur due to defects in the innate immune system, resulting in an aberrant inflammasome and cytokine excess. It differs from autoimmune disorders such as systemic lupus erythematosus (SLE), which occurs due to defect in the adaptive immune system and has auto-antibodies directed towards self-antigens (2).
These are rare disorders that have a striking onset and spontaneous inflammation without any infectious or autoimmune etiology (1). Symptoms include recurrent febrile episodes accompanied by ocular, oropharyngeal, gastrointestinal, dermatological, musculoskeletal, and neurological manifestations. The fever recurs after a fixed interval, and usually resolves spontaneously without any medications (e.g., antibiotics, anti-inflammatory, or immunosuppressive agents) (3). Patients are generally asymptomatic between the episodes, with normal growth and development, but suffer a lot during the attack periods. Although periodic fever syndrome is a rare syndrome, its early diagnosis is essential to improve the patient’s quality of life and decrease the short-term as well as long-term morbidity caused by it.
Table 1 lists the disorders included in PFS and their mode of inheritance. Periodic fever syndrome is characterized by recurrent spontaneous inflammation and elevated acute-phase reactants during and even between the attacks. Symptomatology includes inflammation of serosal surfaces (e.g., pleuritis, pericardial effusion, and joints), neutrophilic skin rashes or urticaria, lymphadenopathy, hepatosplenomegaly, arthritis, involvement of other organs (e.g., muscles and central nervous system), and long-term risk of secondary amyloidosis (4). Periodic fever syndrome can be differentiated from chronic rheumatic disease by its typical recurrence of attacks and symptom-free intervals. Furthermore, it can be confirmed by genetic testing (5). Similar family history can be suggestive of its genetic origin.
| Disorder | Inheritance |
|---|---|
| Unknown inheritance | |
| PFAPA syndrome | None |
| Known inheritance | |
| FMF | AR |
| Cryopyrin-associated periodic syndrome | AD |
| Familial cold autoinflammatory syndrome | |
| Muckle-wells syndrome | |
| Neonatal onset multisystem inflammatory disease | |
| Mevalonate kinase deficiency | AR |
| TRAPS | AD |
| Cyclic neutropenia | AD |
| Pyogenic lesions | |
| Deficiency of interleukin -1 receptor antagonist | AR |
| PAPA | AD |
| Granulomatous lesions | |
| Blau syndrome | AD |
Periodic Fever Syndromes and Their Mode of Inheritance a
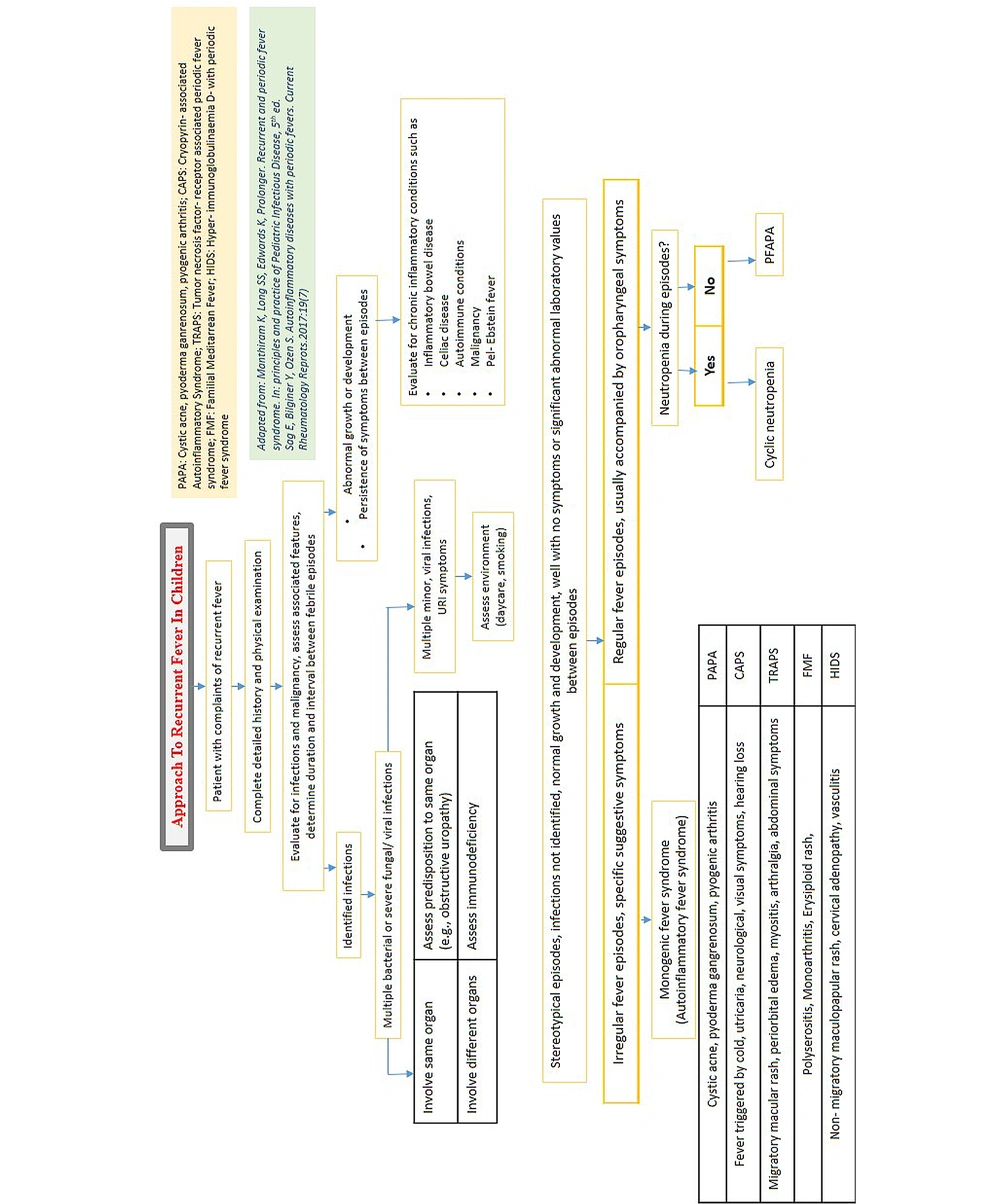
A definite diagnosis is established for many PFS such as familial Mediterranean fever (FMF), mevalonate kinase deficiency (MKD), tumor necrosis factor receptor-associated periodic syndrome (TRAPS), and familial cold auto-inflammatory syndromes (FCAS) after respective detection of mutations in Mediterranean fever (MEFV), mevalonate kinase (MVK), tumor necrosis factor receptor superfamily member 1A (TFRSF1A), and NLRP3/NLRP12 (Nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing) gene, respectively (8). Up until recently, no genetic cause was known for periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA); however, significant familial clustering reported by many studies is suggestive of a genetic predisposition. It has polygenic or multifactorial origin. Multiple low-penetrant gene variants in MEFV or NLRP3 gene are indicative of its polygenic origin (9). Figure 1 displays the approach to diagnosing PFS when a child presents with recurrent fever (10, 11).
Therefore, three cases referring to a super-specialty tertiary care hospital with complaints of recurrent fever and diagnosed with periodic fever syndrome were investigated in our study in order to add more data on this syndrome to the available literature. It is worth mentioning that the diagnosis of these cases was difficult since they were rare and presented with various signs and symptoms, and no easy genetic testing was available at many centers to confirm the diagnosis. Diagnostic criteria for the three cases are shown in Boxes 1, 2 and 3.
| Familial Mediterranean Fever Criteria |
|---|
| Major criteria |
| Typical attacks (recurrent (at least 3 episodes), febrile (rectal temperature ≥ 38°C), and short duration (12 hours to 3 days)) |
| 1- Generalized peritonitis |
| 2- Unilateral pleuritis or pericarditis |
| 3- Monoarthritis (hip, knee, ankle) |
| 4- Fever alone |
| 5- Incomplete abdominal attack |
| Minor criteria |
| Incomplete attacks involving either or both of the following sites. (temperature < 38°C, attack duration longer or shorter than a typical attack (but no less than six hours and no more than seven days), and no signs of peritonitis during the attacks) |
| 1- Chest |
| 2- Joint (other than hip, knee, ankle) |
| 3- Exertional leg pain |
| 4- Favorable response to colchicine |
Familial Mediterranean Fever Criteria a
| Clinical Classification Criteria |
|---|
| Score ≥ 5 points |
| Presence |
| Fever ≥ 7 days (2 points) |
| Fever 5 - 6 days (1 point) |
| Migratory rash (1 point) |
| Periorbital edema (1 point) |
| Myalgia (1 point) |
| Positive family history (1 point) |
| Absence |
| Aphthous stomatitis (1 point) |
| Pharyngotonsillitis (1 point) |
The Eurofever/PRINTO Clinical Classification for Tumor Necrosis Factor Receptor- Associated Periodic Syndrome a
| Classification Criteria |
|---|
| Onset: Early childhood, generally < 5 years |
| Regularly recurring abrupt episodes of fever lasting 5 days, associated with both of the following: |
| Aphthous stomatitis and/or pharyngitis (with or without cervical adenitis) |
| Elevated acute inflammatory markers |
| Completely asymptomatic interval periods (generally lasting less than 10 weeks), benign long-term course, normal growth parameters |
| Exclusion of cyclic neutropenia |
| Exclusion of other episodic syndromes (familial Mediterranean fever, hyper-IgD syndrome, TRAPS, Behcet disease) by family history and the absence of typical clinical features and laboratory markers |
| Absence of clinical and laboratory evidence for immunodeficiency, autoimmune disease or chronic infection |
Modified Marshall’s Classification Criteria for Periodic Fever, Aphthous Ulcers, Pharyngitis and Adenopathy a
2. Case Presentation
In this study, three cases aged nine years referring to our center with recurrent fever case I and complaining of fever persisted for more than a week were reported. It was associated with diffuse erythematous, warm plaque-like lesion of the overlying calf skin and severe calf pain. Examination was performed, and the meningeal signs were found positive. The child was initially thought to have infective meningitis, and, therefore, a lumbar puncture was performed, and intravenous antibiotics were injected. However, the child showed no improvement on a complete course of antibiotics. Neuroimaging was normal.
Case II aged six years, with a complaint of recurrent fever persisting for 4 - 5 days and recurring every 3 - 4 weeks. Examination was performed, and painful ulcerated lesions were observed in the mouth, and multiple cervical lymph nodes were found palpated. The child had received multiple courses of antibiotics from outside. All the infective cultures were negative.
Case III aged four years, with a complaint of recurrent fever persisting for almost 4 - 5 days and recurring every month. Each fever episode was associated with pain and swelling of a single large joint. It subsides spontaneously and appears in different joint in the next episode. There was no residual damage. Examination was performed and the hepatosplenomegaly was determined, and the echocardiogram showed mild pericardial effusion. Further investigation indicated that Brucella titers were positive, but Brucella DNA PCR was negative.
Table 2 shows the clinical features, examination findings, investigations, and final diagnosis of the three cases in greater detail.
| Case I | Case II | Case III | |
|---|---|---|---|
| Age of onset | 9 years | 6 years | 4 years |
| Attack duration | > 7 days | 4 - 5 days | 5 - 7 days |
| Interval between attacks | 6 - 12 weeks | 25 days | 1 month |
| Triggers | None | Upper respiratory tract infection | None |
| Cutaneous manifestations | Diffuse erythematous, warm plaque-like lesion of overlying skin of the calf | Painful ulcerated lesions in mouth (aphthous ulcers in oral mucosa) | None |
| Musculoskeletal manifestations | Severe calf pain | No arthritis | Monoarthritis of large joints (ankle, knee, elbow) in each episode: Non-deforming |
| Abdominal manifestations | None | None | None |
| Eye | None | Conjunctival congestion | None |
| Pleural, pericardial manifestations | None | None | Mild pericardial effusion + |
| Neurological manifestations | Meningeal signs + | None | None |
| Lymph/spleen | None | Bilateral cervical lymphadenopathy (level I & II, 2 X 1 cm); bilateral grade II tonsillar hypertrophy with congestion | Hepatosplenomegaly |
| Anthropometry and development | Normal as per age | Normal as per age | Normal as per age |
| Hematological investigations | Normocytic normochromic anemia; neutrophilic leukocytosis; raised inflammatory markers; CRP 160 mg/dL; ESR 60 mm in 1st hour | Normocytic normochromic anemia; neutrophilic leukocytosis; raised inflammatory markers; ESR- 39 mm in 1st hour; CRP- 98 mg/dL | Normocytic normochromic anemia; neutrophilic leukocytosis; raised inflammatory markers; CRP 185.9 mg/dL; ESR 55 mm in 1st hour |
| Infective workup | EBV, parvovirus B19, borrelia recurrentis, brucellosis, tuberculosis, histoplasmosis, leishmaniasis negative; blood culture sterile; urine culture sterile; CSF examination-8 lymphocytes, protein 60 mg%, glucose- normal, culture sterile | Tuberculosis negative; swab culture from oropharynx- no growth; blood culture- sterile | Brucella abortus antibody titers- reactive 1:80; Brucella melitensis antibody titers- reactive 1:320; Brucella DNA PCR- negative; EBV- negative; tuberculosis- negative; blood culture- sterile |
| Special test | ANA negative; ANCA negative; CECT head- normal; doppler of lower limb- normal; NCV- normal; muscle biopsy- monocytic fasciitis; immunodeficiency workup- negative | ANA negative; immnodeficiency workup- negative | Bone marrow biopsy is cellular, showing hematopoietic elements of all three lineages with normal maturation.no granuloma/atypical cells/hemoparasite seen; anti-CCP- negative; RA factor- negative; ANA – negative; DsDNA-negative; DCT-negative; immunodeficiency workup - negative |
| Genetic results | Negative for pathogenic variants of periodic fever genetic panel | Not done | Heterozygous missense variant in exon 10 of the MEFV gene |
| Diagnosis | Mutation negative tumor necrosis factor receptor- 1- associated periodic fever syndrome (TRAPS) like phenotype | PFAPA (periodic fever, aphthous stomatitis, pharyngitis, adenitis) | FMF |
| Treatment | Pulse steroid followed by maintenance steroid therapy; etanercept | Symptomatic management with NSAIDS and prednisolone 1 - 2 mg/kg single dose | Steroid; colchicine |
| Follow-up duration | 3 years; urinalysis- negative for micro albuminuria | 5 years | 2 years; urinalysis-negative for micro albuminuria |
Comparison of the Cases of Periodic Fever Syndrome
The given cases showed no response to antibiotics, extensive infective, immunodeficiency, rheumatologic, and autoimmune, and malignancy workup was negative. Given the typical periodic self-limiting nature of illness with highly raised inflammatory markers, a differential diagnosis of periodic fever syndrome was considered. After reviewing the literature, the periodicity and symptomatology were found to be consistent with different periodic fever syndromes.
Case I met the criteria for TRAPS according to Eurofever Printo clinical classification criteria (13), but the periodic fever genetic panel was negative. A diagnosis of tumor necrosis factor receptor-associated periodic fever syndrome-like phenotype was established. The child was treated with pulse steroid therapy followed by maintenance steroid therapy. Given sub-optimal disease control, Etanercept was added. The child showed a satisfactory response to the drug and was followed up for three years with no complications.
Case II met the criteria for PFAPA according to modified Marshall’s classification (14). This diagnosis was not confirmed by the genetic test and therefore, final diagnoses of PFAPA were established. All episodes were managed symptomatically with NSAIDS and prednisolone single dose (1-2 mg per kg). The child showed improvement with the treatment.
Case III Child met the criteria for familial Mediterranean fever according to familial Mediterranean fever criteria by Livneh et al. (12). Genetic analysis showed a heterozygous missense variant in exon 10 of the MEFV gene, and a final diagnosis of familial Mediterranean fever was established. The child was treated with pulse steroid therapy followed by steroid maintenance therapy. The child was later given colchicine because of the development of steroid toxicity. The child was then followed up.
3. Discussion
Our cases were afflicted with the rare PFS from an Indian scenario and with its presentation. Familial Mediterranean fever, an autosomal recessive disorder, occurs due to gain-in-function mutations in the MEFV gene that encodes for pyrin. Our patient had a classical history of recurrent febrile episodes with monoarthritis and hepatosplenomegaly during the attack with an otherwise normal inter-febrile period with no positive family history. The genetic workup was positive for the MEFV mutation. The child was initially given steroids and low-dose colchicine, to which the child showed a response. Agarwal and Sharma reported a case of a 16-year-old boy in Jaipur who had five episodes of polyserositis and two episodes of diabetic ketoacidosis over an 8-month period with asymptomatic episodes between the attacks (15). He had no fever, no Mediterranean ancestry, and similar family history. He was diagnosed with FMF based on the clinical criteria. Gicchino et al. also investigated a 13-year-old boy with a history of T1DM for four years who presented with a periodic pattern of fever associated with abdominal pain, chest pain, and arthralgia, and had positive mutations for the MEFV gene (homozygous E148Q mutation) (16). He was initially given colchicine at 1 mg/kg. Arslan et al. documented FMF in a 5.5-month-old child who had three episodes of fever on the 22nd and 71st days as well as at 5.5 months of life with no evidence of infections and a positive family history of FMF in elder sister and recorded homozygous positive for M694V mutations (17). Familial Mediterranean fever has no cure; however, the symptoms can be controlled using NSAID for pain relief and as an anti-inflammatory drug and saline for hydration. Colchicine can be used for mild to severe inflammatory attacks. As for mild attacks, oral colchicine can be applied. In unresponsive cases, intravenous colchicine can be rarely used (18, 19).
Tumor necrosis factor receptor-associated periodic syndrome, an autosomal dominant disorder with incomplete penetrance, occurs due to mutations in the gene TNFRSF1A that encodes for a protein, tumor necrosis factor receptor-1 (TNFR1). This protein is present in the cell membrane and encodes for another protein called tumor necrosis factor (TNF). It sends a signal to the cells to initiate the inflammation. Mutations in the TNFRSF1A gene lead to abnormal production of TNFR1, which may not be released from the cell and may clump there. It is believed to trigger alternate pathways for inflammation. The final diagnosis is reached after performing the genetic analysis. In some cases, however, the genetic study may fail to show the confirmatory genotype due to the phenomenon of somatic mosaicism. Mutations occurring during the late stages of embryogenesis affect only the nongonadal cells and are restricted to a specific population of cells. These mutations may escape detection if screening does not involve the affected cell populations (20, 21). Our patient presented with musculoskeletal (severe calf pain and tenderness), cutaneous (erythematous rash), and neurological manifestations (positive meningeal signs). The genetic workup was negative for the PFS panel and was labeled as mutation negative TRAPS like illness (22, 23). The child was treated with pulse steroid followed by maintenance steroid therapy and later was given Etanercept at the dose of 0.8 mg/kg subcutaneously once a week, to which the child showed a response. Headache is present in 20-25 percent of patients of TRAPS. The disease, associated with the R92Q variant of TNFRSF1A gene mutation, later presents with more headaches and fewer complaints of rash and ocular features at a median age of 5.7 years (24). Hamsen et al. reported the case of a 10-year-old girl who presented with a periodic pattern of fever, skin edema, and abdominal pain (25). All infective workups and the genetic analysis for PFS were negative. There was no family history of PFS. Symptoms partially resolved using prednisolone and etanercept, and the child showed a dramatic response to anakinra.
Periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis syndrome is the most common autoinflammatory disorder in the childhood period, with sporadic onset and no genetic mutations associated with it. The diagnosis is usually clinical, and no genetic test is available for it (26). Our patient presented with classical recurrent episodes of fever, aphthous ulcers, pharyngitis, and cervical lymphadenopathy, and the clinical diagnosis of PFAPA syndrome was established as per modified Marshall criteria after ruling out infective etiology. Our patient was managed with single-dose steroid and symptomatic treatment. Semianchuk reported a case of a 7-year-old boy who had complaints of recurrent episodes of high-grade fever (> 40°C), sore throat, and white spots on his tonsils until the age of two; therefore, he was treated with multiple antibiotics over time and was finally diagnosed clinically with PFAPA after ruling out all the infectious causes (27). Yamahara et al. reported an adult-onset PFAPA syndrome who presented at the age of 37 years with recurrent episodes of high-grade fever, sore throat, aphthous stomatitis, and bilateral enlargement of cervical lymphadenopathy associated with tenderness (28). After ruling out the infective etiology, the diagnosis of PFAPA syndrome was confirmed based on Padeh’s criteria. The patient showed no response to oral medications, and eventually required a tonsillectomy. Multiple investigations performed to find out the cause increased the parents’ financial strains and the child’s emotional stress of needle prick pain. It delays the diagnosis and the proper timely management of the actual disease. The timely diagnosis of PFS is limited by the lack of awareness of pediatricians about its occurrence and the unavailability of genetic testing in most setups. It is essential for a pediatrician to pay close attention to the history of the child and perform a detailed examination when s/he presents with complaints of recurrent fever; it is also necessary for him/her to consider the differentials among periodic fever syndromes if the child shows no response to the first-line antibiotics and if the infective workup is negative. The proper management of PFS patients requires using guidelines. Furthermore, guidelines can assist pediatricians in diagnosing the issue correctly and prescribing effective antibiotic (6).
3.1. Conclusions
In sum, it was recommended that the patients experiencing recurrent fever should be evaluated for PFS even though this syndrome was generally uncommon and rare. As a frequent primary complaint, fever was commonly believed to have an exclusively infectious etiology and was treated with antibiotics. In several cases, multiple courses of antibiotics were given to the patient before a final diagnosis or suspicion of PFS was established. This caused widespread antibiotic abuse, which may have resulted in the emergence of antimicrobial resistance.