1. Background
Sore throat or acute tonsillopharyngitis, often referred to as angina catarrhalis in Central and Eastern Europe, affects mainly children, adolescents and young adults and is one of the most common reasons to consult a family physician (1). While most patients complaining of sore throat have an infection, it has been estimated that fewer than 20% present with a clear indication for antibiotic therapy, i.e., are positive for β-hemolytic streptococcus (2). Since only a small subset of patients suffers from streptococcal infections and even these cases only rarely develop late complications such as rheumatic fever, widespread prescription of antibiotics for sore throat has come under increased criticism e.g., (3-5). These opinions have been supported by the American Academy of Pediatrics, which recommends antibiotic therapy only for children with pharyngitis, evidently caused by group A streptococcus (1). Thus, there is certainly a therapeutic need for new treatment strategies, at least for those without streptococcal infection.
Furthermore, EPs 7630 (EPs® 7630 is the active ingredient of the product Umckaloabo® (ISO-Arzneimittel, Ett-lingen, Germany)), a herbal drug preparation from the roots of Pelargonium sidoides, has been shown to exhibit antiviral (6-8), antibacterial (9) as well as modulating and stimulating effects on the non-specific immune system (9-11), which are based on inhibition of bacterial adhesion to host cells (10), improved phagocytosis and intracellular killing of microbes (11), increased mucociliary clearance (12), interferon-like action (9, 13) and enhancing effects on the production of cytokines (9). The latter two are especially essential for activation of innate defense mechanisms against infections (9, 14). Due to its mode of action, EPs 7630 neither harbors the risk of developing bacterial resistance (10), nor could there be found any indication of risk to develop resistance in viruses (8).
2. Objectives
Meanwhile, a series of placebo-controlled clinical trials have been published, showing the efficacy and tolerability of EPs 7630 in adults and children with acute bronchitis (15-20) and adults with the common cold (21). The outcome measure employed in the trials investigating EPs 7630 in acute bronchitis, the bronchitis severity scale (BSS), comprises of five symptoms indicative of acute bronchitis, namely coughing, sputum production, rales/rhonchi at auscultation, chest pain during coughing, and dyspnea, and is a reliable tool for assessing actual symptom severities in acute bronchitis. A recently published meta-analysis evaluating the results of randomized clinical trials investigating EPs 7630 in acute bronchitis and other RTI stated the superiority of EPs 7630 to placebo in reducing the BSS total score over a treatment duration of 7 days (22).
As an add-on therapy for patients with chronic obstructive pulmonary disease, EPs 7630 led to postponement of exacerbations, reduced exacerbation frequency and antibiotic use (23).
Later, a first double-blind randomized placebo-controlled trial investigating EPs 7630 in children with acute non-group-A beta-hemolytic streptococcus (GABHS) tonsillopharyngitis was published (24). In this trial, EPs 7630 was administered as three daily dosages a 20 drops EPs 7630 over a treatment duration of 6 days. The efficacy of EPs 7630 was measured by assessments of the tonsillitis severity score (TSS) at baseline and on day 4. This symptom severity score comprised of five sub-scores reflecting the nature of the acute non-GABHS tonsillopharyngitis, as a virus-induced inflammatory infection of the upper airways, namely difficulty in swallowing, sore throat, increased salivation, pharyngeal erythema, and fever.
By day 4, the TSS total score reduction was significantly more pronounced in children treated with EPs 7630 compared to those without active medication. Although the TSS total score under EPs 7630 was reduced by 7.1 ± 2.1 points (n = 73) by day 4, the reduction in the placebo group by then was 2.5 ± 3.6 points (n = 70). With a comparably early onset of response, all sub-scores in the TSS improved to about a similar degree. Reductions of TSS total and sub-scores were found to be superior to placebo by day 2 (24). The trial, furthermore, demonstrated that EPs 7630 administered in children with acute non-GABHS tonsillopharyngitis was well tolerated.
We now report on a second trial on this patient group, which was conducted at the same time in order to independently confirm the results obtained by the first trial.
3. Methods
3.1. Design
This study was performed as a prospective, randomized, double-blind, multicenter trial with two parallel groups. An adaptive group sequential design, including up to four interim analyses, allowed for early termination or sample size adjustment in case of continuation (25-28).
3.2. Setting and Ethics
The study was conducted at nine study centers specializing in pediatrics or otorhinolaryngology in Kiev, Ukraine, according to the international Good clinical practice guidelines and the declaration of Helsinki (study registration: ISRCTN27398531).
The protocol and informed consent forms were approved by the local ethics committee and the ministry of health of Ukraine. The legal representative of each patient gave written informed consent prior to enrolment.
3.3. Patients
After approval, consecutive patients seeking treatment of acute tonsillopharyngitis were screened for trial eligibility. Inclusion criteria: children aged 6 to 10 years, acute tonsillopharyngitis (sore throat, catarrhal angina), duration of complaints ≤ 48 hours, negative rapid test for β-hemolytic streptococcus, tonsillitis symptoms score (TSS, details see below) ≥ 8 points, and written informed consent of the patient's legal representative. Exclusion criteria: evidence of lacunar or follicular angina, more than two episodes of tonsillitis within the last 12 months, mandatory indication for therapy with antibiotics (e.g., abscess, septic tonsillitis, status post rheumatic fever, post-streptococcus glomerulonephritis, and chorea minor Sydenham), treatment with antibiotics within 4 months prior to study inclusion, increased hemorrhagic diathesis, severe heart, kidney or liver diseases, immunosuppression, known or suspected hypersensitivity to study medication, concomitant treatment potentially influencing study outcome or known interactions with study medication (e.g., coumarin derivatives), participation in another clinical study within the last 3 months prior to study inclusion, irresponsibility of patients, inability of the patient’s legal representatives to understand nature, importance and implications of the study.
3.4. Treatments and Assessments
Treatment duration according to the protocol was 6 days. Patients were assessed on Day 0 and, if eligible, treated with either 20 drops of EPs 7630 three times daily or placebo. Follow-up assessments were scheduled for day 2, day 4, and day 6. On day 6, individual health checks were scheduled for reasons of safety rather than efficacy determination of the study treatments.
The protocol defined the TSS as the primary efficacy variable, which comprised of the following symptoms, difficulty in swallowing, sore throat, salivation and redness of the throat. At each visit, each symptom had to be scored by the responsible investigator as severe (= 3), moderate (= 2), mild (= 1) or not present (= 0). Finally, a fever score comprising the categories < 37.5°C = 0, 37.5° to < 38.5°C = 1, 38.5° to < 39.5° C = 2, and ≥ 39.5°C = 3 was added, thus creating a possible TSS range between 0 to 15. The TSS data were additionally analyzed categorically as secondary efficacy variables pertaining to different response criteria (TSS Day 4 < 5; change Day 4 in TSS ≥ 5; TSS Day 4 < 5 and change Day 4 in TSS ≥ 5).
Furthermore, the following symptoms, swelling of uvula, throat, tonsils, and lymph nodes, pressure tolerance of lymph nodes, joint pain, headache and other complaints, were assessed using the same scores as for the above symptoms.
On day 4, day 6 or at premature discontinuation, patients (or their legal representatives) were asked to judge the treatment outcome using the integrative medicine outcomes scale (IMOS), including the following categories, “complete recovery”, “major improvement”, “moderate or slight improvement”, “no change” and “deterioration”. Furthermore, patients were asked to estimate the time of treatment effect onset.
Medical history, adverse events and concomitant medication including treatment with paracetamol were recorded.
3.5. Statistics
Change in TSS from day 0 to day 4 was defined as the primary efficacy variable and addressed in hypothesis testing based upon the intention to treat (ITT) set, which comprised of all patients who were randomized, exposed to study medication and provided follow-up information. For sensitivity, this analysis was repeated with the per protocol evaluable set, i.e., those, who completed the study without major protocol violations. These tests were conducted in a confirmatory manner; all other analyses concerning the secondary efficacy variables were descriptive and are to be interpreted accordingly.
Each of the originally planned four interim analyses had confirmatory character and compared the arithmetic means of TSS change on Day 4, using a two-factorial analysis of covariance with the factors treatment and center and the baseline (Day 0) as covariate. Missing values were replaced according to the last observation carried forward (LOCF) principle. The overall type I error was defined as 0.025 and was one-sided. Hence, the adjusted one-sided p-values for the four interim analyses and the final analysis were calculated as α1 = α2 = α3 = α4 = α5 = 0.007907, giving each analysis the same level of significance (25, 26). To investigate the influence of the LOCF method (29), the final data set was also analyzed with recent methods for the handling of missing data (30, 31).
The first interim analysis was scheduled after the first 40 patients had completed the trial, i.e., approximately 20 patients per group. A statistical analysis plan was devised for each interim analysis and finalized based on a data review before unblinding.
The categorical data for the single symptoms and for the patient diary judgments were reclassified based on changes from baseline to last observation using the following categories, “remission”, “improved”, “unchanged”, and “worsened”.
If not stated otherwise, arithmetic means and standard deviation (SD) are given. All data shown refer to the ITT set, which was identical to the safety evaluable set.
4. Results
4.1. Analysis
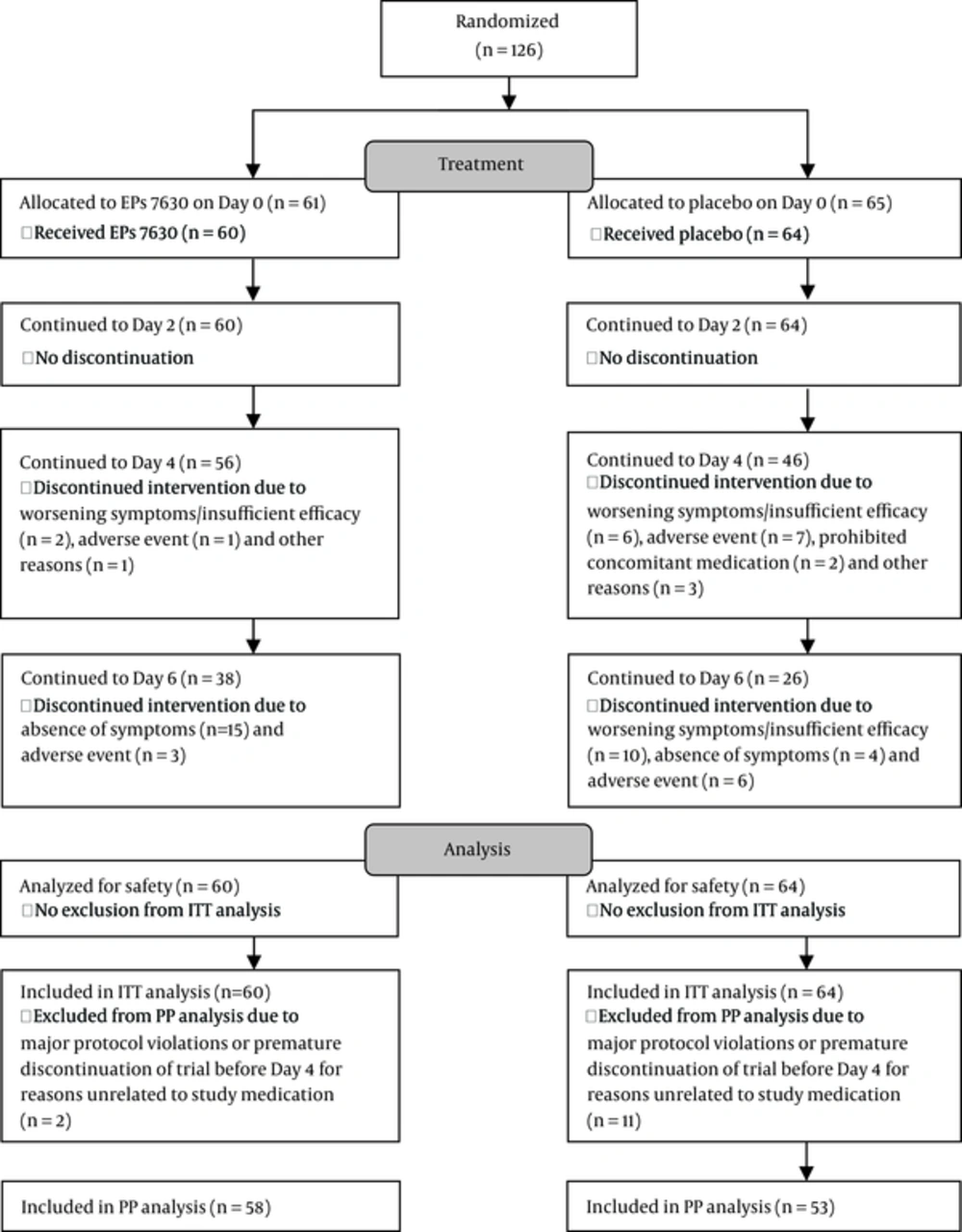
The trial was stopped after the second planned interim analysis showing significant superiority of EPs 7630. The final analysis presented here is based on all patients, including those who were still enrolled at the time of the second interim analysis. Of the 378 patients screened, 126 were found to be eligible and randomized. Two patients did not take any investigational product and were thus not included in any analysis. Hence, the ITT set comprised of 124 patients, of whom 60 were treated with EPs 7630, and 64 with placebo (Figure 1).
Both treatment groups were similar in demographic variables and baseline characteristics (Table 1). The most common prior Ear-Nose-Throat (ENT) diseases were rhinopharyngitis, angina tonsillaris, and otitis media. Only a minority of these infections had been treated with antibiotics.
| EPs 7630 (n = 60) | Placebo (n = 64) | |
|---|---|---|
| Gender: male, No. (%) | 29 (48.3) | 28 (43.8) |
| Age, y | 7.6 ± 1.1 | 7.4 ± 1.2 |
| Height, cm | 129 ± 8 | 130 ± 7 |
| Weight, kg | 26.9 ± 5.1 | 27.2 ± 4.4 |
| Prior ENT infection, No. (%) | 39 (65.0) | 43 (67.2) |
| Prior ENT surgery, No. (%) | 9 (15.0) | 3 (4.7) |
Compliance up to study termination or premature discontinuation was excellent. Only one patient from the placebo group missed a scheduled visit due to change of residency. Three other placebo patients stopped treatment on their own.
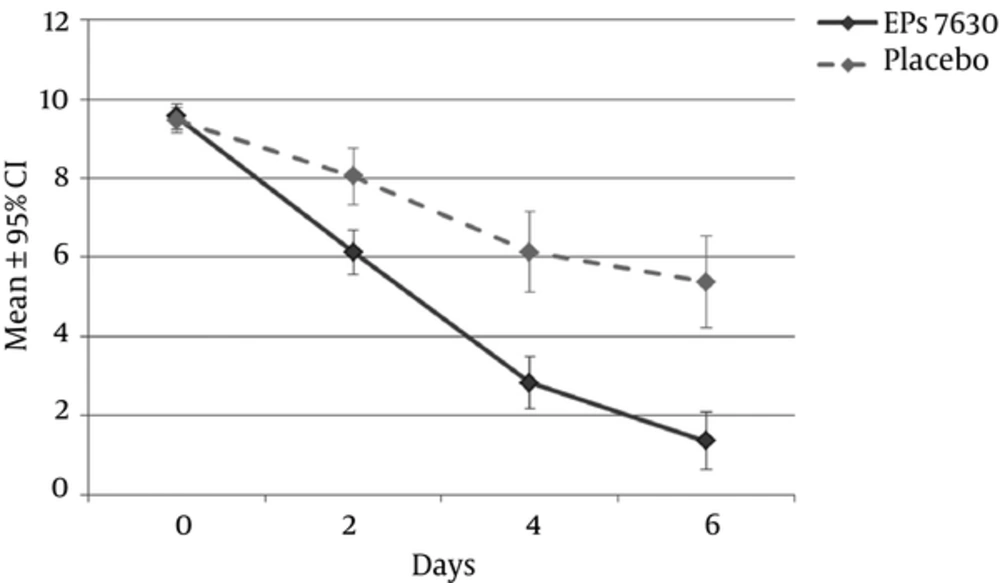
After 4 days of treatment, the TSS had decreased from 9.6 ± 1.2 to 2.8 ± 2.6 points in the active medication group and from 9.5 ± 1.3 to 6.1 ± 4.1 points in the placebo group (P < 0.001). The average point estimates for TSS changes on day 4 indicated improvements after EPs 7630, which were 3.2 points greater than after placebo (95% CI -5.2 to -2.0 points, P < 0.001, P-value of the two-sided covariance analysis). Regarding the recovery process, there was a time-related advantage of 2 days for the EPs 7630 group compared to placebo, with a further substantial increase of this difference towards day 6 (Figure 2). Sensitivity analyses to evaluate the robustness of these findings confirmed the results.
The TSS data were categorically analyzed using three slightly different response criteria (TSS Day 4 < 5; change Day 4 in TSS ≥ 5; TSS Day 4 < 5 and change Day 4 in TSS ≥ 5) and indicated an approximately doubled response rate in the active medication group (85%, 88% and 83% for EPs 7630, and 44%, 47% and 41% for placebo, respectively; P < 0.001 each, p-value of two-sided Fisher”s exact test).
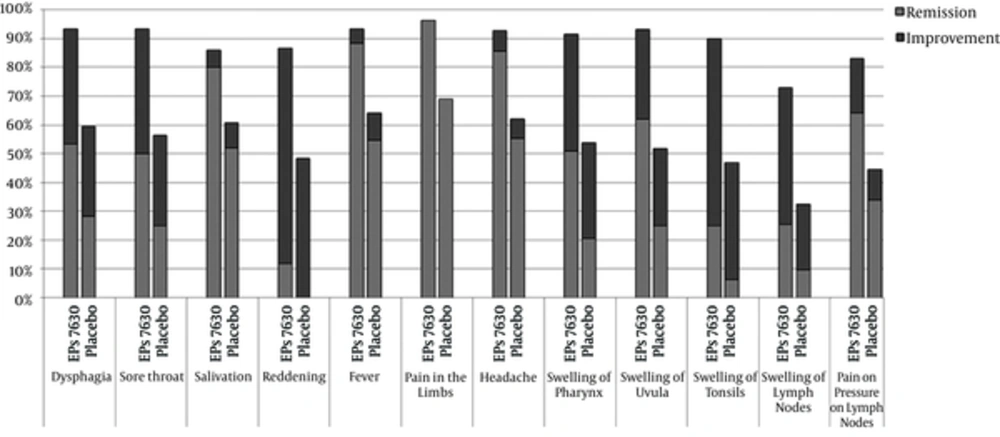
The analysis of individual TSS-relevant symptoms revealed the highest remission rate for fever (Figure 3). Remission and improvement rates of all symptoms were considerably higher under EPs 7630 than placebo (P < 0.012, P-value of the two-sided Fisher’s exact test).
The length of the light gray bar corresponds to the percentage of patients with “symptom resolved” and that of the dark gray bar to the percentage with “symptom improved" on Day 4. The percentages are based on the number of patients, who had the respective symptom on Day 0. The first five symptoms constituted the TSS and ITT.
The majority of EPs 7630 patients judged their health status to be clearly improved by day 4 (57%; IMOS) and were complaint-free by day 6 (80%). The proportion of respective judgments in the placebo group at these time points was 22% and 25% (P < 0.001, two-sided Cochran-Mantel-Haenszel test). Eighty-seven percent of the patients treated with EPs 7630 estimated the onset of treatment effect to have occurred by day 4, whereas 28% considered the treatment effect onset to have occurred by day 2. Furthermore, 30% of the placebo patients reported a treatment effect by day 4 (P < 0.001, two-sided Cochran-Mantel-Haenszel test).
The average consumption of paracetamol (acetaminophen) suppositories during the entire study was significantly lower in the EPs 7630 group than in the placebo group (1.6 ± 1.5 vs. 2.8 ± 2.0, P = 0.001, P-value of the two-sided Wilcoxon test).
4.2. Safety and Tolerability
In total, 21 adverse events (AEs) in 20 patients were documented (5 AEs in 4 patients in the EPs 7630 group and 16 AEs in 16 patients in the placebo group). Most AEs were infections or rather super-infections or symptoms of the same. In one patient treated with EPs 7630, a causal relationship of the AE to study medication could not be excluded but was assessed as unlikely; all other AEs were assessed as unrelated to study medication. Serious adverse events did not occur.
The good safety results were completed by the patients’ and representatives’ final overall judgment of tolerability, which was assessed as “very good” or “good” by 93% of the EPs 7630 patients and 92% of the placebo patients.
5. Discussion
Children with non-streptococcal tonsillopharyngitis are often over-treated with antibiotics (32). As shown in this study, 6-day administration of EP 7630 in pediatric patients with acute non-streptococcal tonsillopharyngitis resulted in improvement that was significantly superior to placebo as confirmed by a clinically relevant decrease of disease symptoms. The comparison of the treatment groups concerning the primary efficacy variable, the TSS total score change, clearly displayed the efficacy of EPs 7630. Therefore, it is important to stress that TSS improvements were based on an overall improvement of acute tonsillitis symptoms. The TSS comprises of most specific symptoms of acute tonsillopharyngitis. Being a localized inflammatory response to viral pathogens, the core symptoms of acute tonsillopharyngitis are reflected by the following TSS items, “sore throat”, “difficulty in swallowing” (pain due to swelling of tonsils and pharynx), “fever”, “hypersalivation”, and “pharyngeal erythema” (reddening) (33-35).
Further categorical analysis of the TSS data showed a roughly doubled response rate to EPs 7630. Remission and improvement rates of all single symptoms were considerably higher under EPs 7630 than placebo, with fever having the highest remission rates of the symptoms constituting the TSS.
A far greater proportion of EPs 7630 than placebo patients judged their health status to be clearly improved and then complaint-free in the course of the study. Also, consumption of paracetamol in the EPs 7630 group was nearly half of that in the placebo group, which indicates a pronounced alleviation of painful inflammatory symptoms until Day 4.
From the patients' estimates of treatment effect onset, it can be deduced that a 50% success rate was achieved up to 3 days earlier with EPs 7630 compared to placebo. This is certainly a clinically relevant difference, as a recent Cochrane review on the effectiveness of antibiotics in upper respiratory tract infections (URTIs) estimated the average benefit in terms of shortened disease to be only 16 hours with antibiotics as compared to placebo (36, 37). As acute tonsillopharyngitis, in particular the variant negative for β-hemolytic streptococcus, is of a self-limiting nature, as evidenced for instance by the placebo group in this trial, the most important aspect from the patient’s perspective might be the more rapid onset of improvements in the actively treated group. These results are in accordance with data from an earlier published clinical trial on EPs 7630 on children with acute tonsillopharyngitis (24), in which the TSS total score had decreased from 10.3 ± 1.2 points to 6.8 ± 2.2 under EPs 7630, and from 9.7 ± 1.4 to 8.2 ± 2.8 points under placebo by day 2. On day 4, this difference had further increased showing a TSS total score reduction of 7.1 ± 2.1 points in the EPs 7630 group vs 2.5 ± 3.6 points in the placebo group.
Generally, clinical trials in self-limiting diseases such as acute non-GABHS tonsillopharyngitis are subject to challenges, as a careful timing of assessments is required in order to obtain reliable data. A comparison of both clinical trials investigating the efficacy of EPs 7630 in this indication suggests the choice of day 4, as the assessment day to be sensible, as results obtained by then display pronounced differences between those children treated with EPs 7630 and those without active medication.
Other Randomized Controlled Trials (RCTs) investigating the efficacy of EPs 7630 in acute bronchitis and the common cold (15-21) likewise demonstrated a reduction of time to onset of symptom alleviation as compared to placebo, thus indicating a beneficial effect of EPs 7630 in RTI and supporting the findings obtained in the presented trial.
Moreover, a meta-analysis of data from an earlier published clinical trial, and the independently obtained results of the trial presented here (22) shows an overall pattern of expedited treatment effect onset and shortened disease duration under EPs 7630 as compared to placebo. This finding could be based on the particular mode of action in EPs 7630, which comprises of antiviral (6-8), antibacterial (9) and immune modulating effects (9-11). The inhibition of the streptococcal invasion of epithelial cells protects the host from microorganisms that evade host defenses and antibiotic treatment, thus preventing recurrent infections. By these means, its mode of action is entirely different from that of classical antibiotics (10). As EPs 7630 does not interfere with bacterial life cycle and metabolism, development of bacterial resistance does not occur. Likewise, it has been demonstrated that EPs 7630 does not show any propensity to induce resistance in viruses (8).
The protocol of this study specifically excluded patients tested positive for β-hemolytic streptococcus because they most likely would have benefited from antibiotics (38). However, many studies on the efficacy of antibiotics in sore throat did not select their target population in such a way, partly because such rapid tests were not available at the time and partly because the authors deliberately wanted to study a broader population possibly reflecting clinical practice more closely (36). An earlier Cochrane review did not find a relevant difference to antibiotics in the (overall rather weak) response to placebo when using a confirmed streptococcus infection as a factor in the model (39). This might be attributable to the lack of precise diagnostic tools at the time. In contrast, the present protocol took special care of this issue.
The overall safety profile of EPs 7630 proved to be favorable. The number of AEs turned out to be considerably higher in the placebo group than in patients who were administered EPs 7630. The obvious difference between placebo and active treatment group with regards to the occurrence of e.g. acute bronchitis, nasopharyngitis and cough in the course of the trial indicates the capability of EPs 7630 in preventing complications and an aggravation of the preexisting acute tonsillopharyngitis due to the antiviral, antibacterial and immunomodulatory effects of EPs 7630 reported earlier (6-14). These findings also confirm the safety results of the formerly published clinical trial of EPs 7630 in children with acute tonsillopharyngitis (24).
5.1. Conclusions
The obvious beneficial effects of EPs 7630 together with its favorable safety and tolerability profile make it a reasonable treatment option for acute non-streptococcal tonsillopharyngitis.