1. Introduction
Factor XIII deficiency (F XIII), the last enzyme in the coagulation cascade, is essential for normal homeostasis. It inhibits fibrinolysis and protects the clot from early breakdown (1). This factor, which is found in plasma, platelets and monocytes (2), is composed of two “A” subunits and two “B” subunits. Sakata et al. (3) first described FXIII deficiency in 1960. Since this first description, up to 200 cases have been reported in the literature (4). Congenital FXIII deficiency is a rare disease but it is considered as one of the most severe hereditary bleeding disorders, with an estimated incidence of about 1 in 106. This disorder affects all races and both genders equally. It is inherited as an autosomal recessive trait, and usually manifests in life as early as infancy or early childhood (5). Most patients with inherited F XIII deficiency, experience severe and lifelong bleeding tendency with a high risk of death in early life because of intracranial hemorrhage.
Bleeding diathesis is severe in cases with inherited F XIII deficiency. The neonate with this disorder is at risk of bleeding immediately after birth (6). Umbilical bleeding in a newborn, which is considered as an important diagnostic clue, occurs in about 80% of the cases and is almost pathognomonic of F XIII deficiency (7). The Iranian tradition of circumcision within 24 hours of birth can lead to severe bleeding.
The incidence of intracranial hemorrhage is higher in F XIII deficiency than in other inherited bleeding disorders (about 30% of all cases). This represents an early life-threatening factor and remains the main cause for the majority of deaths (8). These patients have a life-lasting tendency to bleed into the skin and subcutaneous tissues, which can cause extensive bruises. Bleeding into muscles and around joints are other common characteristics of this disorder, which could sometimes occur even after a minor trauma (6). Strenuous exercise can lead to bleeding in muscles, even in the absence of obvious local trauma. Bleeding from the gums during teething can become a serious problem. However, luckily bleeding from mucous membranes (i.e. epistaxis, gastrointestinal and renal hemorrhage) is a rare feature of this disorder (6).
A few patients have shown a tendency of mild bleeding, which can only be detected when a hemorrhagic procedure is attended, such as a surgical operation (9). Female patients with F XIII deficiency are unable to carry a pregnancy to term and present recurrent miscarriages (10). Wound healing problems have been reported in a minority of these patients (about 14%) (9). The occurrence of keloid in these patients may be due to poor quality of clotting, which leads to abnormally large scar formation (11). This article aimed to elaborate clinical features of a young girl with such severe bleeding after a dental procedure.
2. Case Presentation
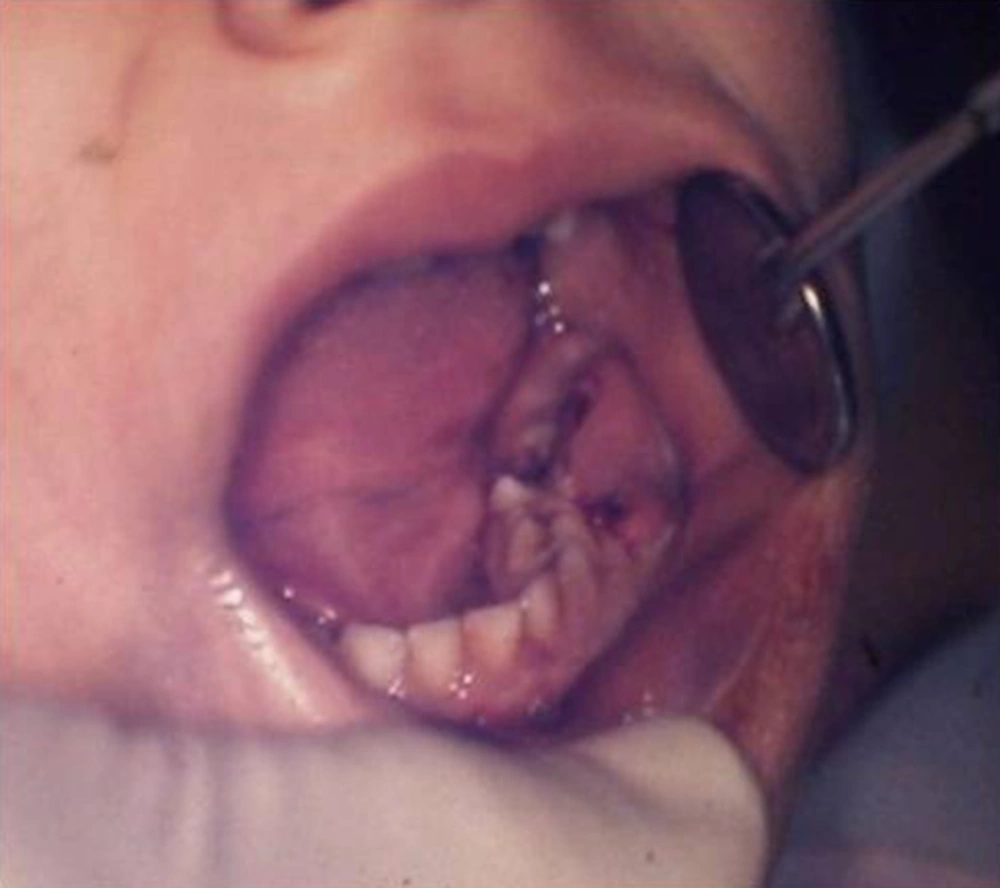
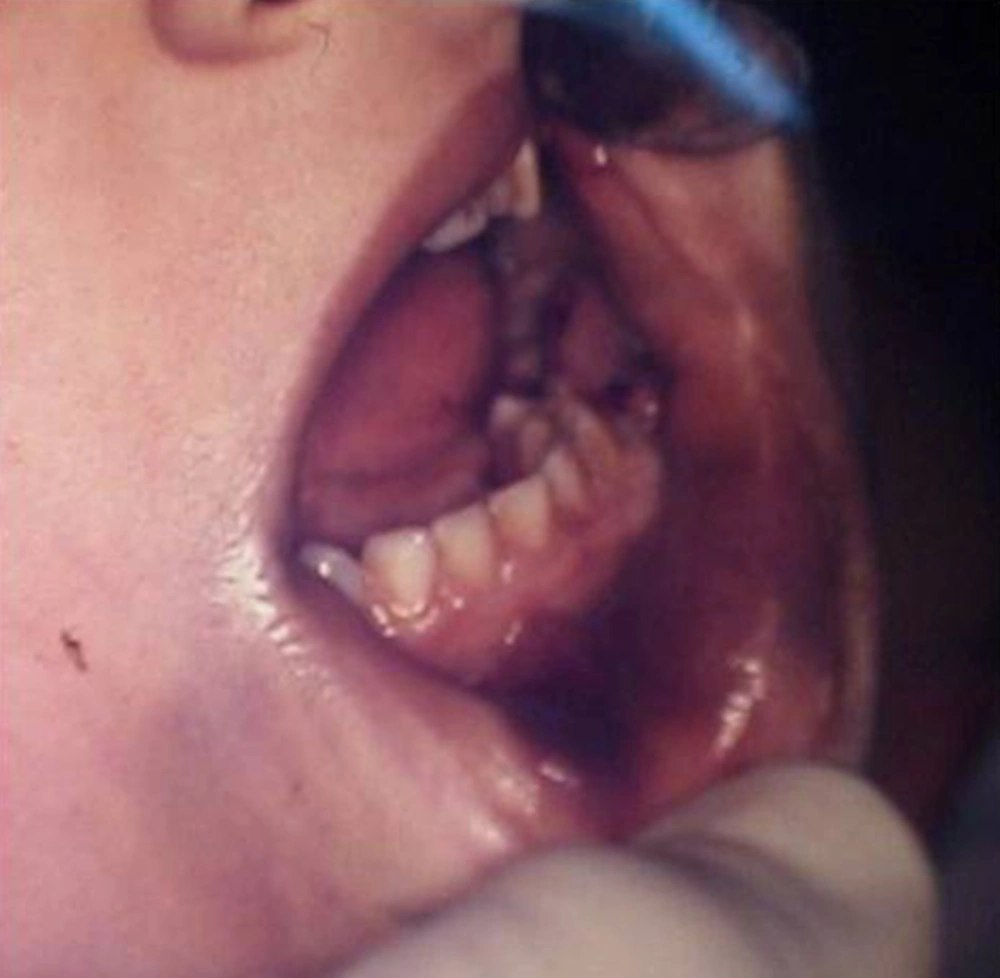
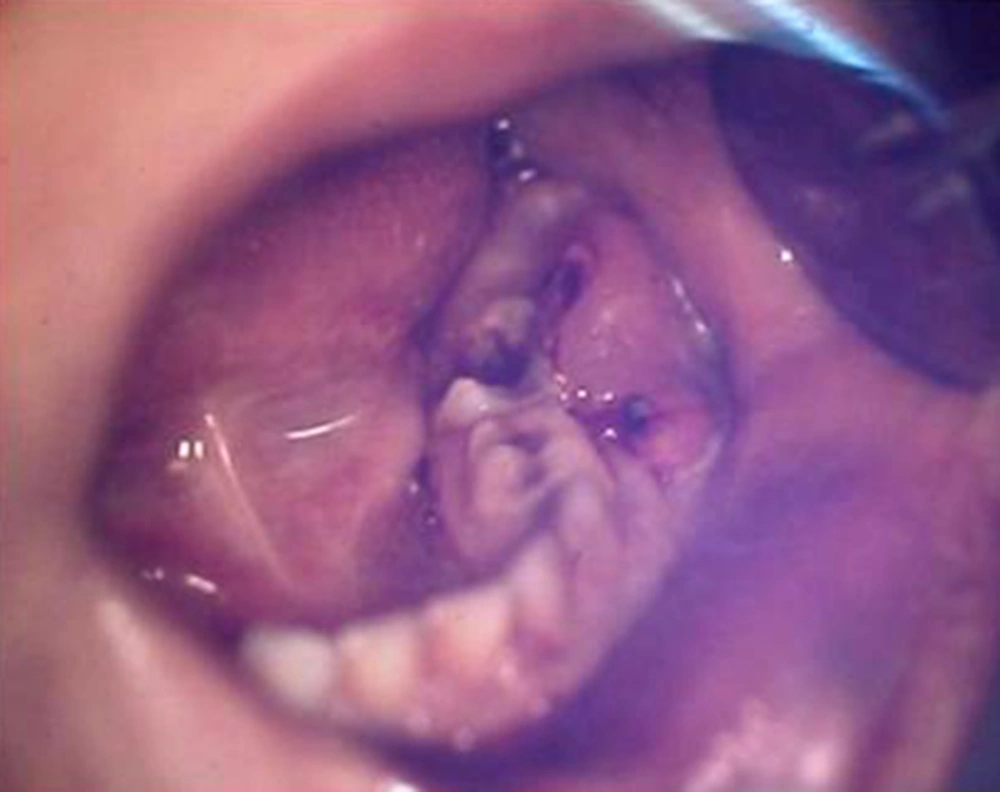
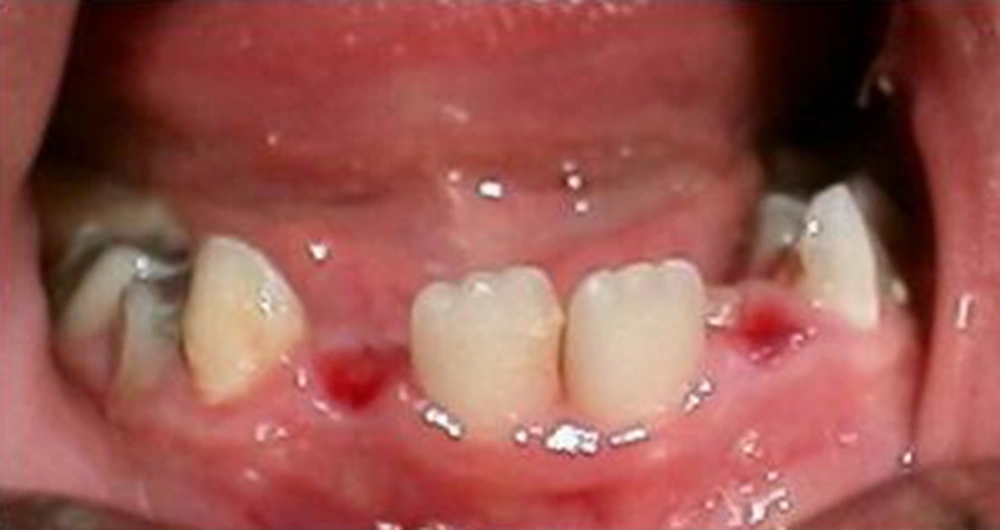
A five-year-old female of middle-eastern origin was referred by a general dentist to a pediatric dental clinic for management of excessive bleeding. The problem started when her dentist conducted an incision at buccal vestibule of carious mandibular left primary second molar, to drain the abscess and reduce the swelling. The patient was pale on arrival to the dental clinic, with signs of malaise, agitation and fear. There was prominent swelling along with a large area of hematoma spanning anteriorly from the buccal vestibule of the first permanent molar, involving the mucus membrane of the lower lip and lingual of the lower left dentition. Crepitus feeling during palpation of the swelling together with oozing of blood from the clot was one of the main clinical findings (Figures 1 and 2).
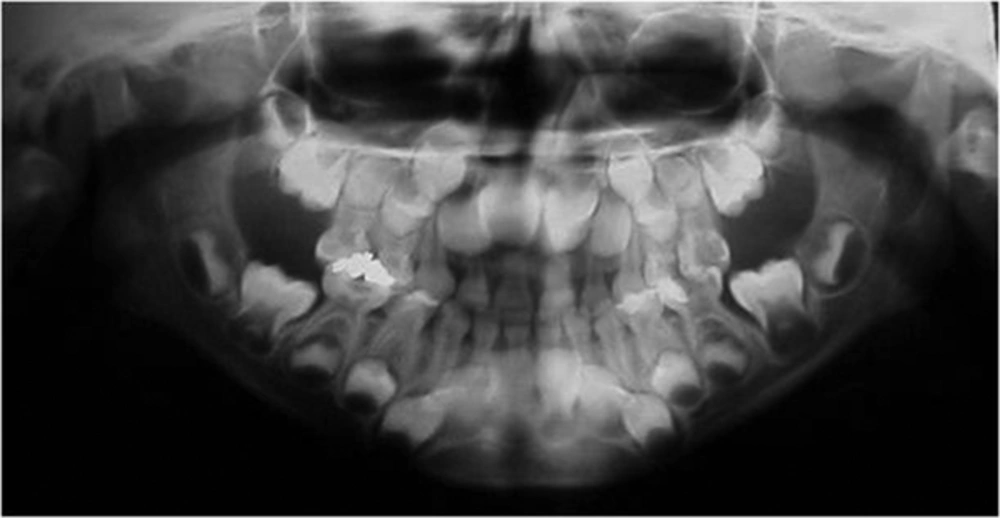
Panoramic radiograph revealed deep carious lesion involving the pulp of the lower left second primary molar with no indication of forcation or apical pathology. Carious lesions involved other primary molars as well with some being treated before with amalgam. The panoramic radiograph also revealed the presence of a supernumerary tooth in the upper left central incisor region (Figure 3).
The patient was reportedly the only child of a non-consanguine marriage. There was no trace of any family history of bleeding disorders. Postnatal history report revealed that umbilical bleeding had taken place, which had lasted up to two weeks. Other historical findings were negative except for occasional but minor ecchymosis due to trauma to her feet or hands.
2.1. Management
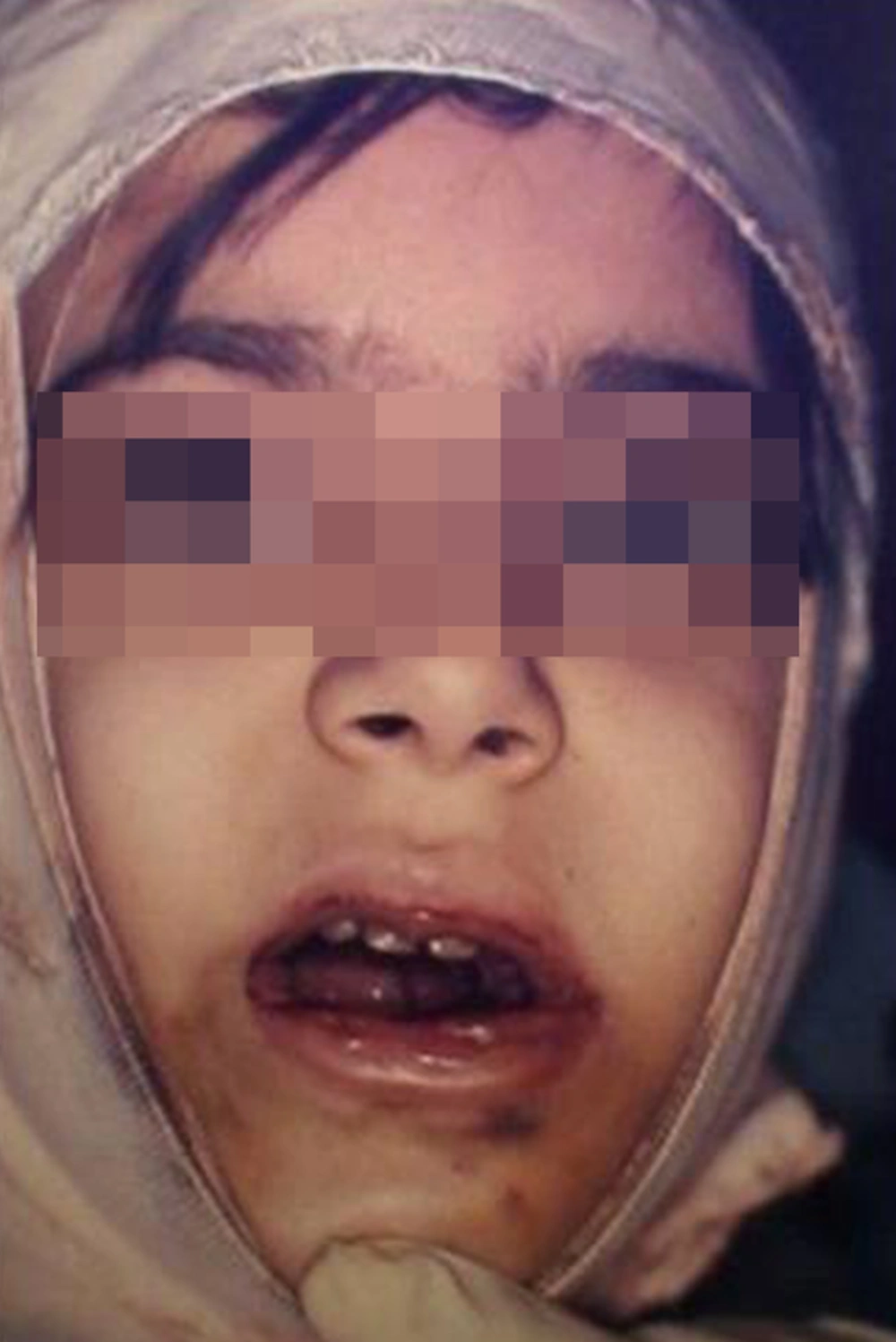
The patient was referred to a local children’s hospital in order to control the bleeding. The following steps were carried out in line with bleeding control. An initial application of a sterile gauze pad over the extracted site after removing all the clots followed by an extra oral bandage of the face in order to apply pressure and control the bleeding (Figure 4).
Crystalloid solution was infused to substitute the amount of blood loss and to control the tachycardia. In the next step, a laboratory blood test was conducted. Hematologic examinations included the bleeding time (BT), prothrombin time (pt), partial thromboplastin time (PTT), and platelet count. All of which were found to be in the normal range. There was slight reduction in number of Red Blood Cells (RBC = 3.8 × 103), Hemoglobin (HGB = 10.6 g/dL) and Hematocrit (HCT = 31.0%), which could be explained by the excessive Blood loss. As the lab test results were not indicative of any clear bleeding disorder, the patient was hospitalized for further consultations and specific blood tests. Clot solubility test was then recommended at this stage for detection of a possible factor XIII problem. The result of the test revealed significant decrease in the level of Factor XIII (sub-unit A was less than 5%).
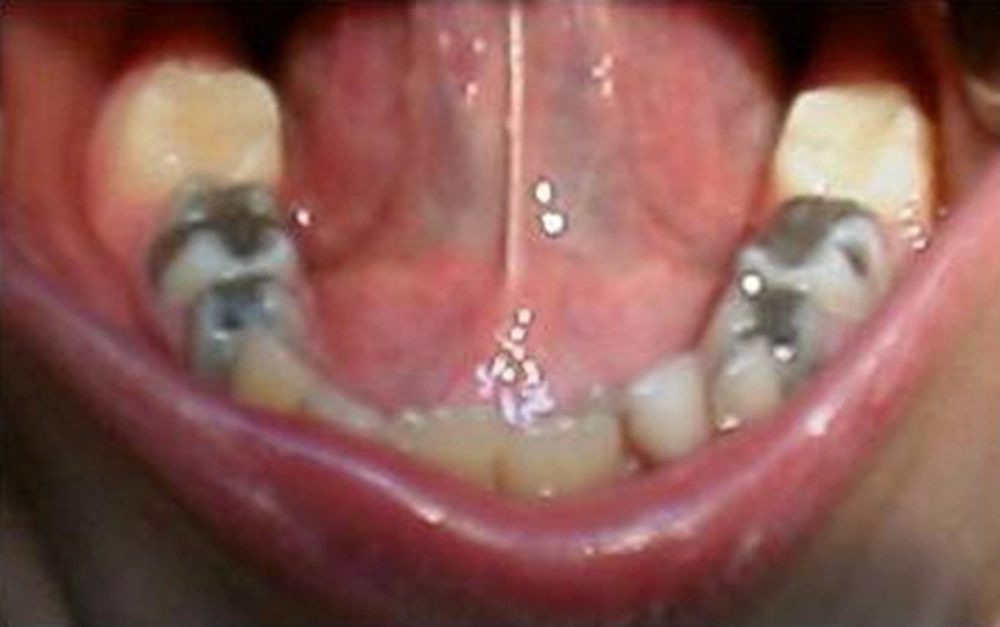
On the basis of the clinical and laboratory findings, a diagnosis of factor XIII deficiency was eminent and confirmed by the hematologist. The patient subsequently received six bags of cryoprecipitate (containing F XIII) in order to obtain accurate homeostasis acceptable for such individual with severe bleeding from the oral cavity. If not controlled, it may have led to blood clot aspiration. Such preparation will allow further treatment on the subsequent days. The patient was discharged two days later with good general condition. Cryoprecipitate was prescribed for the patient, as a prophylactic measure, with an order of being administered prior to every medical or dental procedure with bleeding potentials. The patient was scheduled for a comprehensive dental treatment planning. Carious lesions were restored and Preventive Resin Restorations (PRR) were performed on several of the first permanent molars (Figures 5 and 6).
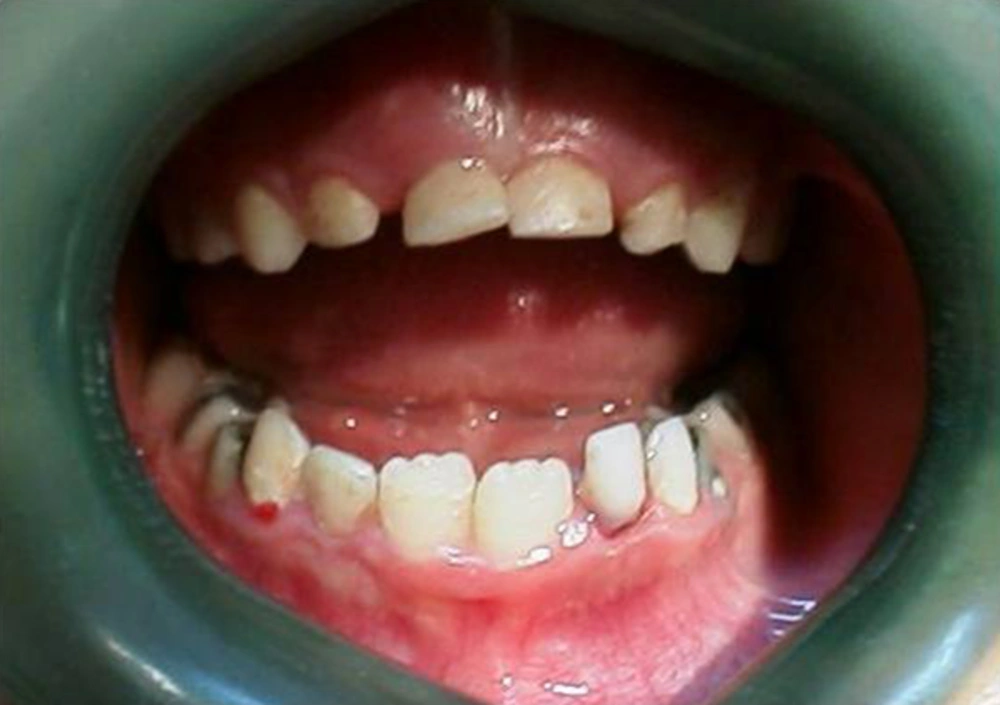
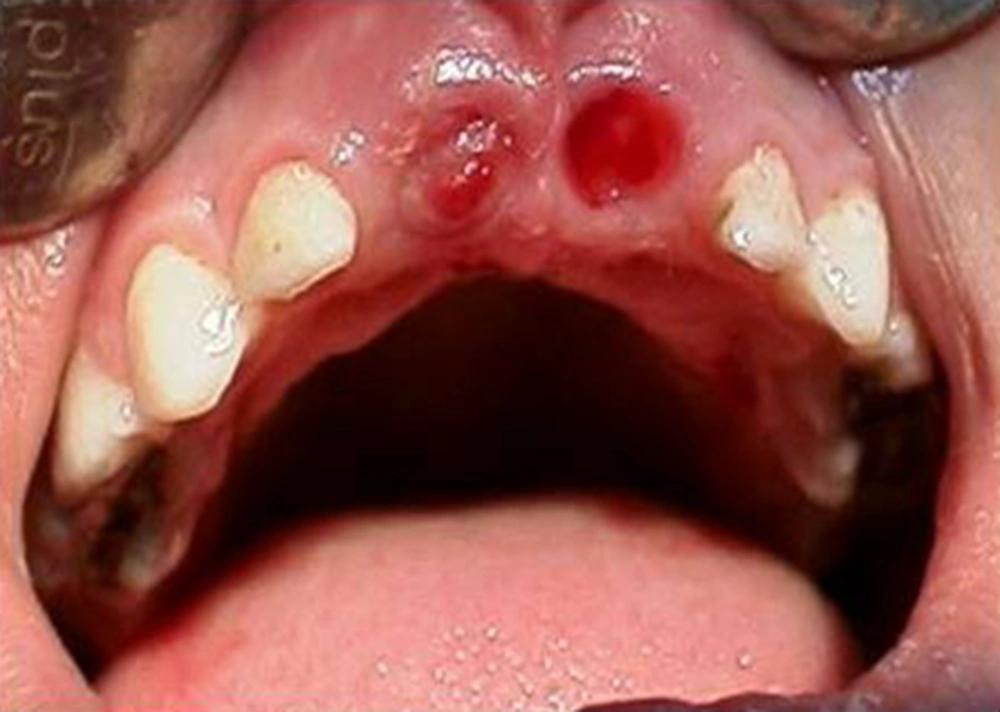
A year and a half later, the mandibular primary central incisors were exfoliated with reportedly no bleeding complication. At seven years of age, the child returned with complaint of oozing gingival sulcus of loose maxillary primary centrals and mandibular primary laterals (Figure 7).
The involved teeth were scheduled for extraction and the patient was referred to the hematologist for appropriate preparation. Teeth were then extracted without any Problem (Figures 8 and 9).
The supernumerary tooth was also scheduled at the same time for removal while it was still safe to do so. The patient was scheduled for a regular follow-up visit and proper dental and hematologic management, as this was believed to guarantee appropriate patient care.
3. Discussion
As standard clotting tests are normal in cases with F XIII deficiency, due to the clotting end point being unaffected by the absence of F XIII, their clinical diagnosis becomes highly critical. However, if noted from the quality of clot, which is abnormal, such entity will easily be identified and plenty of strategies can be arranged to prevent its complications. In these circumstances the diagnosis will not be missed when the quality of clotting is assessed (6). Standard clot solubility test is the screening test for F XIII deficiency in the urea and in some instances monochloroacetic acid measure (12). A definite diagnosis is then made on the basis of functional and immunological assays (7). For many years, whole blood, fresh frozen plasma or a placental F XIII concentrate have been used successfully in the treatment of F XIII deficiency. More recently, a plasma- derived pasteurized concentrate marketed under the name of Fibrogammin-P (Centeon Pharma GmbH, Marburg, Germany) has also become available on the market. Since very low levels of F XIII in plasma are sufficient to control bleeding, and as the in vivo half-life of F XIII is long (11 - 14 days) after infusion, prophylaxis is considered as a simple and practical step (13). It is believed that any level of FXIII within 3 to 10% of the mean value in the normal population, i.e. 0.03 - 0.1 IU/mL, is sufficient to prevent spontaneous hemorrhage (14-17). Although rare, F XIII deficiency is a remarkable disorder because of its serious bleeding manifestations. In particular, the majority of the cases have life-threatening symptoms early in life. However, there are reports of cases in the literature with problems that had not been detected until a later step and following severe bleeding as a sequel of a minor surgery (7).
The current patient was not diagnosed as an F XIII deficient case until the age of five years, despite prolonged umbilical bleeding during her neonatal period similar to the reported case by Anwar et al. (6). However, the key to early diagnosis is considered as the presence of serious bleeding manifestations, especially intracranial hemorrhage, which can easily be prevented if noted on time through prophylactic F XIII concentrate administration. Vigorous replacement therapy seems to be helpful in controlling the tendency of miscarriage in F XIII deficient pregnant females (6).
Decreased level of RBC*, HGB** and HCT*** may be due to excessive amount of blood loss after the first severe bleeding episode. A positive history of umbilical hemorrhage together with the clinical symptoms, led the hematologist to perform the F XIII assessment in order to confirm the diagnosis. As mentioned earlier, F XIII deficiency is an autosomal recessive disorder. This indicates that both parents must be carriers of the defective gene to pass it on to their children. In this patient, the negative familial and/or consanguinity history may attribute the disorder to mutations in F XIII type A gene. Recent studies have proved that such mutations are the most common causes of F XIII deficiency (15). Unlike this scenario, the case reported by Killick et al. (16) highlighted the importance of seeking familial history of bleeding disorders prior to surgery in the neonatal period, particularly if the parents are consanguineous.
Whole blood, fresh frozen plasma, stored plasma and cryoprecipitate are considered as the adequate sources of F XIII and have commonly been used successfully in the treatment of F XIII deficiency in the recent years (6). Fibrogammin-P is a recently available F XIII concentrate and is derived from the plasma of healthy donors. The methods of preparation of Fibrogammin-P have been described by Karges and Metzner (17). Patients are given 1000 units of concentrate every five to six weeks. A vial of concentrate contains 250 units, which is dissolved in 4 mL of water. Therefore, the patient receives 16 mL of the dissolved concentrate, intravenously for one to two minutes. This raises the plasma F XIII activity level to about 30-35% of average normal levels while the process of coagulation remains normal for five to six weeks.
As young individuals are more active and therefore more prone to injury than adults, it has been recommended that they should receive the concentrate at four-week intervals. However, Miloszewski and Losowsky (18) found six-week intervals as adequate in the prevention of hemorrhage in their patients. Although fibrogammin-P is the product of choice, due to its unavailability, cryoprecipitate (containing F I, F VIII, F XIII and Von Willebrand Factor) was infused to control bleeding. In order to control bleeding before the dental procedure Factor XIII 10 - 20 U/Kg concentrate was used as prophylactic therapy every four to six weeks in order to have adequate plasma level (19-22). As routine, it is recommended for all F XIII-deficient patients to receive prophylactic treatment immediately after confirmation of the diagnosis due to high risk of intracranial hemorrhage. From the dental management point, since hemorrhagic diseases are usually diagnosed at an early childhood stage, child dental clinics are possibly the first to see the manifestation of these disorders. In such cases, an accurate work up and correct laboratory tests and diagnosis could not only control and prevent the hemorrhage but also most of the dental procedures could be delivered to such individuals in a very safe and normal manner, similar to the case of this report.
3.1. Conclusion:
Patients with F XIII deficiency are uncommon, but could face serious life threatening issues following a simple dental procedure. It is important to note that umbilical hemorrhage does provide a diagnostic clue and should lead to a clot solubility test and F XIII assay early in life in order to make the parents and physicians including dentists, prepared for unexpected events.