1. Background
Celiac disease (CD) is an autoimmune disorder of the small intestine, identified by serological and histological characteristics. The disease is predominant in females, and its prevalence is estimated to be around 1:71 in children and 1:357 in adults (1-4). This disorder may present with gastrointestinal symptoms such as diarrhea, abdominal pain, nausea, and vomiting, or extraintestinal manifestations, including failure to thrive, anemia, osteoporosis, delayed puberty, and neuropathy (5). Gluten consumption in genetically susceptible individuals causes an enteropathy leading to villous atrophy and impaired absorption of nutrients (6). In fact, the presence of human leukocyte antigen (HLA) haplotypes of DQ 2 and DQ8 is a major risk factor for the incidence of CD (7), increasing the risk of the disease among the first-degree relatives of patients, especially their siblings (8, 9). The familial occurrence of the disease is reported to range from 2.8% to 22% (10). Therefore, screening CD patients’ family members and their siblings, even if they are asymptomatic, is of great importance (8, 11). However, due to the absence of typical gastrointestinal symptoms of CD in children, most (90%) of them may remain undiagnosed (6, 12). Since the risk of growth failure due to CD has been shown to be relatively high in younger children, the diagnosis of CD is of special significance in pediatric patients (13).
The serologic tests that identify anti-tissue transglutaminase IgA antibodies (tTG-IgA) are useful to evaluate patients with suspected CD and are known as the first diagnostic step in this condition (6). Endoscopy and duodenal biopsy evaluation are the gold standard to confirm the diagnosis of CD in highly suspected patients (14). In terms of histopathological features, duodenal mucosal lesions in CD patients are classified according to the modified Marsh classification (Marsh I, II, and III (a, b, c)), according to which a normal intestinal mucosa is considered to be flat (15, 16). The late diagnosis of the disease and leaving patients untreated may lead to various complications like osteoporosis, infertility, cancer, etc. (5). In regions with a high prevalence of CD, nationwide guidelines are crucial for the early detection and treatment of patients in order to prevent potential drastic complications, including growth retardation in children (13).
Our country, Iran, is among the regions with a high prevalence of CD. A meta-analysis showed that the prevalence of CD among Iranians is about 2% (17). However, there is a scarcity of data on the epidemiological features of CD in Lorestan province, Western Iran.
2. Objectives
Owing to the high concordance of CD among the siblings of affected individuals and the importance of the early diagnosis of the disease in these people to prevent its long-term complications, as well as due to the scarcity of data on CD in our province, we aimed to evaluate the prevalence of CD among the siblings of pediatric CD patients in Lorestan province, Iran, in 2020.
3. Methods
3.1. Study Design and Participants
This cross-sectional study was conducted on 140 siblings (aged 1 to 27 years old) of pediatric patients with confirmed CD (based on clinical presentations, as well as serological and histological findings (referred to the Celiac Disease Clinic of Shahid Rahimi Hospital of Khorramabad city, Lorestan province. Entry criteria included being a sibling of a CD patient, agreement to participate in the study and the absence of gastroenteritis and upper respiratory infections at the time of testing. Exclusion criteria were having chronic infections, already being diagnosed with CD, and IgA deficiency. The minimum sample size was estimated to be 138 individuals according to the following formula. All children who met the inclusion criteria were enrolled until the final sample size was achieved.
3.2. Ethical Considerations
This study was approved by the Research Ethics Committee of Lorestan University of Medical Sciences with the ethical code of IR.LUMS.REC.1398.250. The importance of the disease and the role of screening tests among first-degree relatives, especially the siblings of CD patients, in the timely diagnosis of the condition were explained to parents. Written informed consent forms were signed by parents. Checklists were designed to collect the data anonymously, and the participants were assured that their personal information would remain confidential.
3.3. Serological Screening
Peripheral blood samples from the siblings of children with confirmed CD were collected by a lab technician. Then the serum samples were separated using centrifugation and screened for anti-tissue transglutaminase (anti-tTG) IgA antibodies. Also, total IgA level was measured by an enzyme-linked immunosorbent method using a generic assay kit. For all the participants in this study, anti-tTG IgA antibody was measured at the same laboratory using the same kit. Concentrations of anti-tTG IgA ≥ 30 IU/mL were considered positive.
3.4. Confirmation of CD by Endoscopy
Subjects with tTGA positivity underwent endoscopic biopsy taking for histopathological evaluations to confirm the diagnosis. Increased intraepithelial lymphocytes (IELs), crypt hyperplasia, and villous atrophy were considered the characteristic features of CD, which were then classified according to the modified Marsh classification (Marsh I, II, and III (a, b, c)) (14-16).
3.5. Questionnaire for Data Collection
Upon enrollment, we retrieved the demographic and clinical characteristics of the subjects, like age, sex, height, weight, place of residence, and gastrointestinal and non-gastrointestinal symptoms, into a researcher-made checklist. Weight loss, as a non-gastrointestinal manifestation, was defined as losing ≥ 5% of body weight within 30 days or losing ≥ 10% of body weight within 180 days. Children aged ≥ 12 years answered the questionnaire mainly by themselves and otherwise, with the support of their parents.
3.6. Data Analysis
The data were analyzed using IBM SPSS Version 22 (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). The measures of central tendency and dispersion, as well as tables and graphs, were used to describe quantitative data. The Chi-square test was used to examine the relationship between categorical variables. P-value < 0.05 was considered statistically significant.
4. Results
4.1. Demographic Characteristics
In the present study, 140 siblings of children with CD in Lorestan province of Iran were screened for the disease. The mean age of the subjects was 12.6 ± 6.6 years, with a minimum age of 1 year and a maximum age of 27 years. Sixty individuals (42.9%) had an age below 10 years, 34 (24.3%) were between 10 and 14 years old; 30 (21.4%) were between 15 and 19 years old, and 16 (11.4%) were ≥ 20 years old. In terms of gender, 77 (55%) of the subjects were girls, and 63 (45%) were boys. In terms of the place of residence, most of the participants resided in Khorramabad city (47.8%) (Table 1).
| Variables | Absolute | Relative | Cumulative |
|---|---|---|---|
| Place of residence (city) | |||
| Khorramabad | 67 | 47.8 | 47.8 |
| Aleshtar | 14 | 10 | 57.8 |
| Noorabad | 8 | 5.7 | 63.5 |
| Kuhdasht | 27 | 19.2 | 82.7 |
| Rumeshkan | 11 | 7.8 | 90.5 |
| Aligudarz | 5 | 3.6 | 94.1 |
| Borujerd | 3 | 2.1 | 96.2 |
| Azna | 3 | 2.1 | 98.3 |
| Poldokhtar | 2 | 1.7 | 100 |
4.2. Serologic Findings
Anti-tTG IgA was positive (≥ 30) in 12 subjects (8.6%). Out of the 12 subjects with positivity for anti-tTG IgA, eight (66.7%) were girls, and four (33.3%) were boys. Nine of them (75%) were ≤ 15 years old, and three (25%) were ≥ 15 years old. Also, seven out of the 12 subjects (58.3%) were living in Khorramabad, two (16.7%) in Aleshtar, one (8.3%) in Noorabad, one (8.3%) in Kuhdasht, and one (8.3%) in Aligudarz.
4.3. Histological Features
Among 12 subjects with positivity for anti-tTG IgA who underwent duodenal biopsy, the Marsh stage II was detected in two (16.7%), IIIa also in two (16.7%), IIIb in two (16.7%), and IIIc in six (50%) people. This distribution of different Marsh categories had no significant relationship with the serum level of anti-tTG IgA antibodies (P = 0.319, Table 2).
| Marsh Stage | tTG Ab Levels | P-Value | ||||
|---|---|---|---|---|---|---|
| 30 to 99 | 100 to 199 | 200 to 299 | 300 ≤ | Total | ||
| Marsh II | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 0.319 b |
| Marsh IIIa | 0 (0) | 0 (0) | 1 (50) | 1 (50) | 2 (100) | |
| Marsh IIIb | 0 (0) | 0 (0) | 2 (100) | 2 (100) | 2 (100) | |
| Marsh IIIc | 3 (50) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 6 (100) | |
a Values are expressed as No. (%).
b Chi-square test
4.4. Clinical Features of Newly Identified Patients
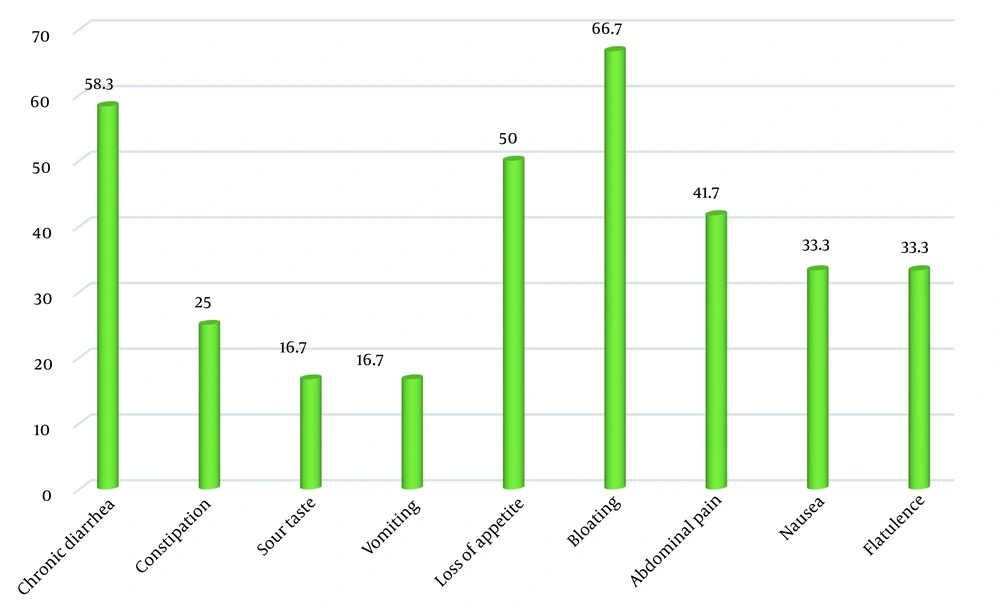
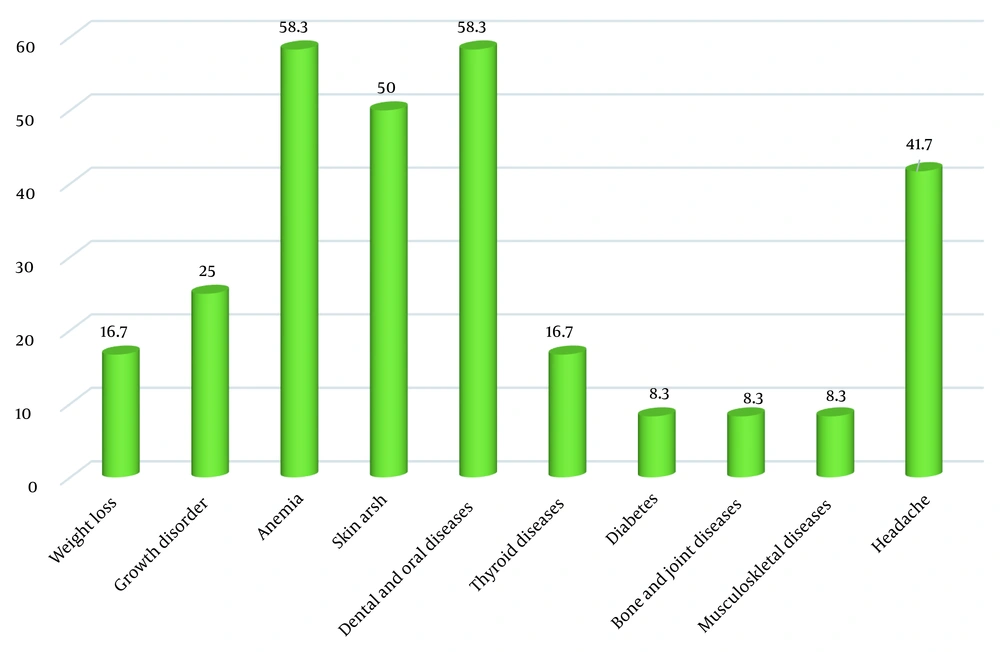
The most common gastrointestinal symptoms of CD patients identified through this screening process were bloating (66.7%) and chronic diarrhea (58.3%). The most common extraintestinal symptoms in these newly-diagnosed patients were anemia (58.3%) and dental and oral problems (58.3%) (Figures 1 and 2).
5. Discussion
In the present study, 140 siblings of children with CD in Lorestan province were screened for the disease. The results showed a prevalence of 8.6% of CD among these individuals. The prevalence of CD among the relatives of CD patients has been widely investigated; however, the epidemiological patterns vary greatly in different geographical regions. Dogan et al. (18), in a study in Turkey, demonstrated that 7.25% of the siblings of CD patients were diagnosed with CD. Grover and colleagues (19), in a study in North India, reported a prevalence of 15.6% for CD among the siblings of confirmed cases. A similar study conducted by Bardella et al. (10) on an Italian population revealed a significantly higher prevalence of silent CD among the siblings of affected individuals. In a recent study, 23.8% of the patients’ siblings were diagnosed with CD.
In our study, 66.7% of the siblings who tested positive for anti-tTG IgA were girls, and 75% of whom were ≤ 15 years old. Tapsas et al., in a study on a pediatric population, reported that the majority of the patients identified through screening were between five and 14.9 years old (20). This is consistent with the findings of our study. The higher susceptibility of females to CD has also been reported in several studies (21-23). Nevertheless, Bardella et al. (10) found that male siblings of CD patients were affected in 40.9% of cases, while this rate was 26.9% among female siblings. The female predominance may be justified by a combination of genetic factors, such as gene variants residing on X chromosome, as well as other underlying factors, including pregnancy and menstruation, which affect intestinal permeability (23).
In the present study, the typical gastrointestinal symptoms of CD in the newly-identified patients included bloating, chronic diarrhea, and abdominal pain, which are mainly caused by villous atrophy in the small intestine, which was in accordance with previous research findings. Also, CD can present with non-gastrointestinal signs and symptoms, from a slight elevation in transaminases to hepatic failure and even liver cancer (24). The most common extraintestinal symptoms in our patients were anemia, dental and oral problems, skin rashes, and headache. It has been reported that the presence of extraintestinal manifestations is associated with more severe villous atrophy (25). Previous studies have also reported a variety of extraintestinal symptoms in CD patients, including growth retardation as the most frequent extraintestinal manifestation in pediatric populations (26). Children with growth failure have been assumed to be younger and have lower hemoglobin and higher levels of autoantibodies (13). Other studies have reported anemia, fatigue, and headache as the most common extraintestinal manifestations in children with CD (26-28).
Some manifestations of CD such as vomiting and anemia are assumed to occur more frequently in patients with higher histological grades (i.e., Marsh IIIb and IIIc) (29). Our results showed that half of the newly-diagnosed patients were in the Marsh IIIc stage, indicating disease progression to the final stages of the disease, increasing the possibility of the presence of other CD-related complications.
Overall, genetic predisposition (carrying HLA DQ2 and/or DQ8 haplotypes) is an essential risk factor for the incidence of CD (30). However, despite the high risk of CD among the close relatives of patients, the concordance of CD varies from 49 to 83% among monozygotic twins. Furthermore, non-HLA genetic factors have been confirmed to contribute to the incidence of CD. In western societies, about 40% of the overall population carry one or both of HLA-DQ2/HLA-DQ8 haplotypes; however, only 1% of these people develop CD (31). These findings suggest a role for environmental factors in the development of this disease (9, 32). Therefore, screening the close relatives of known CD cases, even if they are asymptomatic, is crucial to prevent the progression of the disease to late stages and modify environmental risk factors contributing to disease aggravation in high-risk groups (33-35).
5.1. Limitations
A number of limitations can be noted for this study. Some parents of CD patients did not agree to have their healthy children screened for CD, so they were not included in the study. Furthermore, as the studied population mostly consisted of children, we had difficulty collecting their subjective symptoms.
5.2. Conclusions
In conclusion, we found a high prevalence (8.6%) of CD among the siblings of CD patients in Lorestan province of Iran. Since the early diagnosis of CD can lead to its better management, particularly in pediatrics, it is recommended to perform screening tests for patients’ family members, especially their siblings, as soon as a patient is diagnosed. This approach can help prevent serious complications such as growth failure. It is advisable to conduct similar studies in other provinces of the country to help the health care system better allocate services to susceptible individuals.