1. Background
Gestational diabetes mellitus (GDM) is the recognition of glucose intolerance, which first develops during the second and third trimesters of pregnancy and usually disappears after delivery (1, 2). Its prevalence is increasing mainly due to the advanced maternal age, delayed childbearing, and the rise in obesity (3, 4). According to a recent update by the international diabetes federation, hyperglycemia during pregnancy affects 16.2% of live births. GDM accounts for 75 - 90% of the pregnancies complicated by hyperglycemia (5). It is not only associated with increased long-term metabolic risks in the off-springs (6) but can also cause diverse cardiac complications such as the fetal cardiac hypertrophy, diastolic dysfunction, and asymmetric ventricular septal hypertrophy (7). Notably, as cardiac disorders are more common in neonates of the pregestational diabetic mothers, cardiac structural and functional abnormalities, including myocardial hypertrophy and congenital heart disease, are three times more common in infants of mothers with gestational diabetes (IGDMs) than those born to normal mothers. However, there are differences between these two categories regarding the degree of stability and the severity of cardiac involvement. Furthermore, CHD in these infants is five times higher than that in normal pregnancies (8). GDM is mainly associated with hypertrophic cardiomyopathy (HCM), characterized by ventricular wall thickening and asymmetric interventricular septal hypertrophy (9). Although it is usually transient (might disappear between 6 - 24 months after birth) and might not lead to permanent complications, some infants of poorly-controlled diabetic mothers experience serious complications such as congenital heart failure (CHF) due to outlet obstruction (3).
QT interval is the time interval between the beginning of the Q wave in the QRS complex and the end of the T wave in the electrical heart cycle in an electrocardiogram (ECG) (9). Corrected QT (QTc=QT/√ (RR in seconds)), calculated by Bazett’s formula, is another electrocardiographic criterion and is considered normal in the term infants if it is less than 400 milliseconds. Longer QT might also be suggestive of ventricular hypertrophy (10). In an ECG trace, the QT complex represents ventricular depolarization and repolarization, and its values change according to ventricular thickness (11). QT dispersion (QTD), which is defined as the difference between the maximum and minimum QT intervals in 12 leads of an ECG, indicates repolarization heterogeneity. Hypertrophic cardiomyopathy is associated with longer QTD, and this increment is accompanied by a higher risk of sudden arrhythmias and unexpected death (12, 13).
The antenatal evaluation of the cardiac structure by echocardiography and electrocardiography is mandatory (8). Echocardiography could be used as a helpful modality for screening cardiac problems in IGMDs, as the mean values of the septal thickness measured by echocardiography, according to the previous study, was higher in IGDMs compared with the control group (P < 0.001) (3).
2. Objectives
Considering the growing trend of the GDM prevalence, the shortage of evidence on its cardiac complications in neonates, and more accessibility to electrocardiography than to echocardiography in most centers, the present study mainly aimed to investigate the possible correlation between QTD and echocardiographic indices in term neonates with and without maternal GDM. This study also aimed to examine the correlation of QTD with ventricular septal hypertrophy and other echocardiographic findings.
3. Methods
In this analytic case-control study, 38 IGDMs as the case and 38-term neonates with healthy non-diabetic mothers as the control group were included by adopting convenient sampling. They were referred to the heart clinic affiliated with 17 Shahrivar Hospital, Iran, from March 2019 to November 2019. The two groups were matched based on the age and weight. All included neonates were healthy ones born to the mothers with gestational diabetes. Those with maternal systemic diseases such as familial hyperlipidemia, preeclampsia, eclampsia, hypertension, thyroid, heart, kidney, and liver diseases, as well as with smoking, alcohol, or drug abuse history, were excluded from the study. Since electrolyte disturbances cause transient quantifiable changes in the ECG, infants with hypo or hypercalcemia as well as hypo and hyperkalemia were also excluded from the study. Moreover, neonates with a history of exchange transfusion and Intrauterine growth restriction (IUGR) were not enrolled in the study. Electrocardiography (ECG) was performed for all infants, and a standard 12-lead ECG was recorded for ten seconds for all patients by a nurse with the same device (Samsung ECO7, South Korea). All electrocardiographic data were interpreted by the same physician. QT, QTD, and corrected QT (QTc) values were calculated manually. Normal QTc was considered between 350 and 400 milliseconds (3). An echocardiographic assessment was performed by a pediatric cardiologist using the same device (MeCA406i MEDIGATE, South Korea). The echocardiographic parameters, including ejection fraction (EF), interventricular septal thickness (IVSTD) at the end of diastole, left ventricular end-systolic and diastolic diameters (LVESD, LVEDD), and left ventricular posterior wall thickness (LVPWT) were measured. IVST at the end of diastole (IVSTD) was measured from the tip of the mitral valve, and values>6 mms were suggestive of hypertrophy (3). Normal and specific values for the above criteria were not determined in infants due to the dramatic changes in heart structure. EFs equal to 55% and higher were considered normal. The age, sex, weight, and cardiac parameters, including QT (max and min), QTD, QTC, IVSTD, LVEDD, LVESD, LVPWT, and EF were recorded and compared in the two groups.
3.1. Ethical Considerations
This study was approved by the ethics committee of the Vice-Chancellor of Research at Guilan University of Medical Sciences (Code: IR.GUMS.REC.1397.399).
3.2. Statistical Analysis
Data were analyzed using IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, N.Y., USA), and were reported by number, percent, mean, and SD. The normality distribution of the quantitative variables was evaluated by performing the Schapiro Wilk test. The Mann-Whitney U test, independent t-test, and Spearman correlation coefficient were conducted to analyze the quantitative data, and chi-square and Fisher’s exact test were performed to compare the qualitative results. P-value < 0.05 was considered significant.
4. Results
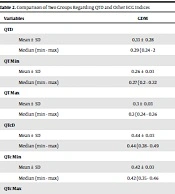
In this study, the neonates' mean age and weight were 4.18 ± 1.3 days and 3418.16 ± 549.24 grams, respectively. As for the gender of the participants, 68.4% and 47.4% of the infants in the case group and control group were female, respectively. There was no significant difference between two groups in terms of age, weight, and sex (P = 0.244, P = 0.172, and P = 0.06, respectively) (Table 1). According to Table 2 which compared QT indices in the two groups of neonates, QTc min in the case was significantly higher than that in the control group (P = 0.046). The two groups had no statistically significant difference regarding other parameters (P > 0.05).
| Variables | GDM | Non-GDM | Total | P-Value |
|---|---|---|---|---|
| Age, d | 0.96 | |||
| 3 | 16 (42.1) | 15 (39.5) | 31 (40.8) | |
| 4 | 9 (23.7) | 7 (18.4) | 16 (21.1) | |
| 5 | 6 (15.8) | 8 (21.1) | 14 (18.4) | |
| 6 | 3 (7.9) | 4 (10.5) | 7 (9.2) | |
| 7 | 4 (10.5) | 4 (10.5) | 8 (10.5) | |
| Total | 38 (100) | 38 (100) | 76 (100) | |
| Age | 3.97 ± 1.18 | 4.34 ± 1.38 | 4.18 ± 1.3 | 0.244 |
| Sex | 0.063 | |||
| Male | 12 (31.6) | 20 (52.6) | 32 (42.1) | |
| Female | 26 (68.4) | 18 (47.4) | 44 (57.9) | |
| Total | 38 (100) | 38 (100) | 76 (100) | |
| Weight, g | 3504.4 ± 18.5 | 3331.8 ± 90.5 | 3418.1 ± 549.2 | 0.172 |
aValues are expressed as No. (%) or mean ± SD.
| Variables | GDM | Non-GDM | Total | P-Value |
|---|---|---|---|---|
| QTD | 0.806 | |||
| Mean ± SD | 0.33 ± 0.28 | 0.28 ± 0.03 | 0.31 ± 0.20 | |
| Median (min - max) | 0.29 (0.24 - 2) | 0.28 (0.23 - 0.36) | 0.28 (0.23 - 2) | |
| QT Min | 0.274 | |||
| Mean ± SD | 0.26 ± 0.03 | 0.27 ± 0.03 | 0.27 ± 0.03 | |
| Median (min - max) | 0.27 (0.2 - 0.32) | 0.27 (0.22 - 0.35) | 0.27 (0.2 - 0.35) | |
| QT Max | 0.229 | |||
| Mean ± SD | 0.3 ± 0.03 | 0.31 ± 0.03 | 0.3 ± 0.03 | |
| Median (min - max) | 0.3 (0.24 - 0.36) | 0.3 (0.24 - 0.38) | 0.3 (0.24 - 0.38) | |
| QTcD | 0.141 | |||
| Mean ± SD | 0.44 ± 0.03 | 0.43 ± 0.03 | 0.44 ± 0.03 | |
| Median (min - max) | 0.44 (0.38 - 0.49) | 0.43 (0.37 - 0.49) | 0.44 (0.37 - 0.49) | |
| QTc Min | 0.046 | |||
| Mean ± SD | 0.42 ± 0.03 | 0.41 ± 0.03 | 0.42 ± 0.03 | |
| Median (min - max) | 0.42 (0.35 - 0.46) | 0.41 (0.34 - 0.46) | 0.42 (0.34 - 0.46) | |
| QTc Max | 0.288 | |||
| Mean ± SD | 0.46 ± 0.03 | 0.45 ± 0.03 | 0.46 ± 0.03 | |
| Median (min - max) | 0.47 (0.41 - 0.5) | 0.46 (0.40 - 0.5) | 0.46 (0.40 - 0.5) |
According to Table 3, the case had a lower EF (62.76 ± 0.52) compared to the control group (64.21 ± 2.18) (P = 0.011). IVSTD was also significantly higher in the case compared to the control group (P = 0.05).
| Variables | GDM | Non-GDM | Total | P-Value |
|---|---|---|---|---|
| EF | 0.011 | |||
| Mean ± SD | 62.76 ± 0.52 | 64.21 ± 2.18 | 63.49 ± 2.45 | |
| Median (min - max) | 65 (60 - 65) | 65 (60 - 70) | 65 (60 - 70) | |
| LVEDD | 0.414 | |||
| Mean ± SD | 12.46 ± 2.78 | 12.02 ± 1.78 | 12.24 ± 2.33 | |
| Median (min - max) | 12.25 (5.8 - 19) | 11.9 (8.9 - 16) | 12.1 (5.8 - 19) | |
| LVESD | 0.503 | |||
| Mean ± SD | 8.8 ± 2.33 | 8.48 ± 1.86 | 8.64 ± 2.1 | |
| Median (min - max) | 8.6 (3.2 - 14.9) | 8.3 (5.4 - 12.8) | 8.5 (3.2 - 14.9) | |
| LVPWTD | 0.132 | |||
| Mean ± SD | 4.23 ± 1.28 | 3.86 ± 0.73 | 4.04 ± 1.05 | |
| Median (min - max) | 4.15 (2.4 - 7) | 3.8 (2.2 - 5.5) | 3.9 (2.2 - 7) | |
| IVSTD | 0.05 | |||
| Mean ± SD | 5.08 ± 1.74 | 4.43 ± 1.04 | 4.76 ± 1.46 | |
| Median (min - max) | 5 (1.2 - 8.9) | 4.3 (2.6 - 7.8) | 4.75 (1.2 - 8.9) |
The Spearman correlation coefficient revealed no significant linear correlation between QTD and other parameters in the two groups (Table 4). According to the results from multivariate correlation analysis, QTD was significantly correlated with LVEDD (β = 0.053, P = 0.001) and LVESD (β = 0.042, P = 0.015). Regarding the high dependence of LVEDD and LVESD with QTD, tolerance and VIF were 0.356 and 0.281, respectively. Since there were almost large values of these factors, it was better not to enter both of them in the same model. By eliminating the LVESD in the model, LVEDD was recorded as the only significant index associated with QTD (β = 0.025, P = 0.001).
| QTD | GDM | Non-GDM | Total | ||
|---|---|---|---|---|---|
| Spearman correlation coefficient | EF | r | 0.182 | 0.052 | 0.101 |
| P-value | 0.274 | 0.755 | 0.387 | ||
| N | 38 | 38 | 76 | ||
| LVEDD | r | 0.183 | 0.086 | 0.138 | |
| P-value | 0.270 | 0.607 | 0.236 | ||
| N | 38 | 38 | 76 | ||
| LVESD | r | 0.237 | 0.033 | 0.141 | |
| P-value | 0.151 | 0.844 | 0.223 | ||
| N | 38 | 38 | 76 | ||
| LVPWTD | r | -0.030 | 0.078 | 0.029 | |
| P-value | 0.859 | 0.641 | 0.8 | ||
| N | 38 | 38 | 76 | ||
| IVSTD | r | -0.077 | -0.208 | -0.179 | |
| P-value | 0.645 | 0.210 | 0.121 | ||
| N | 38 | 38 | 76 | ||
| Age | r | 0.120 | -0.301 | -0.080 | |
| P-value | 0.472 | 0.066 | 0.493 | ||
| N | 38 | 38 | 76 | ||
| Weight | r | 0.003 | 0.018 | 0.011 | |
| P-value | 0.987 | 0.914 | 0.924 | ||
| N | 38 | 38 | 76 |
According to Table 5 and despite the significantly lower amount of QTD in boys than in girls from the case group (P = 0.025), the difference was not statistically significant in the control group (P = 0.246). In addition, no significant difference was observed between QTD regarding the sex (P = 0.424).
| Diabetic Mother | No. | Mean ± SD | P-Value a |
|---|---|---|---|
| Case | 0.025 | ||
| Male | 12 | 0.2692 ± 0.03147 | |
| Female | 26 | 0.3569 ± 0.33643 | |
| Control | 0.264 | ||
| Male | 20 | 0.2890 ± 0.02954 | |
| Female | 18 | 0.2800 ± 0.03395 | |
| Total | 0.427 | ||
| Male | 32 | 0.2816 ± 0.03133 | |
| Female | 44 | 0.3255 ± 0.26024 |
a Independent t-test
5. Discussion
The incidence of DM is increasing and can induce diverse complications. According to the World Health Organization, its incidence may increase to seven million by 2030 if no effective measures are taken. Interestingly, there is an increased risk of congenital malformations in IGDMs compared to the general population (14-16). The relative risk of fetal cardiac involvement in pregnancies complicated with GDM is 2.5 - 10 times higher than that in normal pregnancies (8). Therefore, this study evaluated two groups of the term infants with and without maternal GDM based on the ECG and echocardiographic findings. Our study showed significantly lower EF and higher IVSTD in the case compared to the control group. Although the exact mechanism by which maternal GDM could affect the fetal heart is still unknown, many epidemiological studies have reported a strong association between GDM and the relative increase in fetal congenital heart disease (8, 15). Maternal GDM might lead to hyperinsulinemia and increased insulin receptors in the fetal heart. This, in turn, can be associated with myocardial cell hyperplasia and hypertrophy due to the increased fat and protein synthesis. Moreover, an increased fetal cardiac wall thickness results in the ventricular diastolic dysfunction and might be presented with heart failure (8).
Maternal GDM is associated with a significant increase in the thickness of the intraventricular septum, which could be detected in the echocardiographic assessment of IGDMs (17). In a study by Al-Biltagi et al., a significant increase was observed in the septal/posterior LV wall thickness in IGDMs compared to those in the control group, which was consistent with our study result. The echocardiographic findings of their study showed that all IGDMs had impaired ventricular systolic and diastolic function. Furthermore, pulmonary venous pressure in the systolic state was significantly increased in the IGDM group compared to the control group (18). Consistent with our study result, the findings from the studies by Ren et al., Esmaeeli et al., and Hasmasanu et al. revealed a higher proportion of septal hypertrophy in IGDMs compared to the newborns of healthy mothers. They also found significant difference between the two groups regarding QTD (3, 19, 20).
Gestational age and infant weight were not adjusted in most of the previous relevant studies; however, our results demonstrated higher cardiac wall thickness after adjusting the given variables between groups. Considering the incidence of macrosomia in IGDMs and the possible relationship between body weight and cardiac muscle mass, matching the two groups in terms of weight may have been a strong point of the present study.
QT interval indicates the ventricular depolarization/repolarization time and could be affected pathologically by cardiac wall thickness. Meanwhile, QTD as a difference in the ventricular myocardial recovery periods is expected to be increased in patients with hypertrophic cardiomyopathy (21). In our study, the case group had higher QTc min than the control group. According to cardiac electrophysiology, an increase in all QT-dependent parameters is expected when the thickness of the heart muscle increases. Although other ECG parameters (e.g., QT min, QT max, QTD, …) were higher in the case compared to the control group, no statistically significant difference was detected between the two groups. Despite septal hypertrophy in the case group, Bagheri et al. found no significant difference between IGDMs and the control group in terms of QTc. Furthermore, they found no relationship between septal hypertrophy and QT prolongation, which was consistent with our study results (21, 22). Previous studies have also shown that hypertrophic cardiomyopathy increases all electrocardiographic parameters such as QTD, QT min, and QT max (3, 23).
The main limitations of our study were its small sample size, disregard of the type of mothers' medical intervention (regimen versus insulin), and failure to consider the maternal HGA1c as a contributing factor. Therefore, it was recommended that a study with a larger sample size should be conducted by removing the confounding factors and considering the type of maternal diabetes management. It was also suggested that the cut-off relevant ECG index should be determined to refer the neonate for further echocardiographic evaluation in future studies if a relationship between ECG parameters and echocardiography indices was found due to the more availability of the ECG.
5.1. Conclusions
A proportion of septal hypertrophy was detected in IGDMs compared to infants of healthy mothers without any correlation with ECG indices. This involvement was accompanied by relatively decreased EF verified by echocardiography. Most ECG findings, including QTD values, had no linear relationship with echocardiographic parameters except for LVEDD and LVESD.