1. Background
The World Health Organization (WHO) declared a pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in March 2020. Coronavirus disease 2019 (COVID-19) presents mainly as a lower respiratory tract infection; however, several systems may be involved in this disease (1). The morbidity and fatality rates of COVID-19 are directly and strongly associated with the age of affected patients and risk factors, as the population ≥ 50 years old usually presents with a higher rate of morbidity and mortality (2, 3). The number of infected adults increased during the pandemic. Also, a growing rate of affected children was reported as the first COVID-19-infected infant presented with fever in the Hubei province of China on January 26, 2020 (4). The clinical manifestations of affected children vary from asymptomatic and mild upper respiratory tract symptoms to severe pneumonia and respiratory failure (5)—most of the symptomatic cases present with fever, cough, diarrhea, and sore throat. However, PCR results of nasopharyngeal swap specimens are not always positive for COVID-19 (6).
Beta-thalassemia (BT) is the most frequent inherited disease in Mediterranean countries (7, 8). The amount of beta chain deficiencies resulting in the variability of ineffective hematopoiesis in the bone marrow has categorized the BT patients into two groups of dependent (DBT) and non-dependent (NDBT) blood transfusion (9). The severity and complication of COVID-19 can differ in this population because of a deficiency in the beta chain in their hemoglobin. More frequent transfusion (more than ten times/year), older age, splenectomy, and iron overload (10-12) may make these patients prone to COVID-19 and related complications (11). Karimi et al. (12) reviewed the potential risk factors of COVID-19 infection in BT patients, including iron overload, oxidative stress, underlying chronic liver disease, cardiac complications, and adrenal insufficiency. The adverse effects of iron overload and anemia, which affect end organs, especially in older ages, can lead to a higher risk of complications (12). However, another cohort study with a small sample size in Italy showed that BT patients had mild to moderate COVID-19 with complete recovery despite the BT-associated risk factors (13).
According to some studies, beta-thalassemic patients could develop immunity against COVID-19 as the beta chain, a potential target of this virus, could be either absent or less prominent in these cases. However, there are not sufficient studies to confirm this hypothesis. Due to the limited information and resources of the previous studies, the clinical manifestations, transmission patterns, and complications of SARS-CoV-2 have not yet been fully defined in BT patients.
2. Objectives
In this study, we aimed to determine the serological status of BT patients against COVID-19 and investigate the risk factors of COVID-19 complications in this specific population.
3. Methods
This cross-sectional study was carried out in September 2020-February 2021 at Ali-Asghar Children’s Hospital, Iran, one of the major centers for managing thalassemia cases. All the major and intermedia β-thalassemia patients dependent on blood transfusion and had consent (filled by the patients or their guardians) were enrolled in this study. Demographic characteristics, including age, gender, thalassemia type, history of splenectomy, and blood type, were recorded in a checklist. We put the blood group as a variable because some studies showed the association of this variable with the risk of COVID-19 and consequent complications.
Furthermore, the enrolled patients were examined for anti-COVID-19 IgM and IgG to determine their serological status. The patients declared any exposure to confirmed COVID-19 cases. Finally, they were asked if they had any symptoms compatible with COVID-19, such as cough, fever, and anosmia in the past six months. Patients with a history of confirmed COVID-19 diagnosis (positive PCR) were recorded. At the time of the current study, PCR tests for COVID-19 were limited in Iran. Therefore, only some patients could perform this test, and serology tests were very useful in distinguishing positive cases. In addition, COVID-19 vaccination was not started in Iran at that time. Consequently, we had no positive serology cases because of vaccination. The subjects were excluded only if they did not agree to complete the serologic tests.
The data were statistically analyzed using SPSS 25. Mean, and standard deviation was used to describe quantitative variables. Prevalence and percentage were utilized for qualitative variables. Moreover, a t-test was used to compare the quantitative variables, while analysis of variance (ANOVA) was applied to assess qualitative variables. P-value<0.05 was considered statistically significant.
4. Results
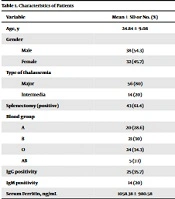
Seventy cases were enrolled in this study, 38 (54.3%) and 32 (45.7%) of whom were female and male, respectively. The mean age of the patients was 24.84 ± 9.08 (range: 7 - 43) years. Demographic and laboratory characteristics of the patients are shown in Table 1.
| Variable | Mean ± SD or No. (%) |
|---|---|
| Age, y | 24.84 ± 9.08 |
| Gender | |
| Male | 38 (54.3) |
| Female | 32 (45.7) |
| Type of thalassemia | |
| Major | 56 (80) |
| Intermedia | 14 (20) |
| Splenectomy (positive) | 43 (61.4) |
| Blood group | |
| A | 20 (28.6) |
| B | 21 (30) |
| O | 24 (34.3) |
| AB | 5 (7.1) |
| IgG positivity | 25 (35.7) |
| IgM positivity | 14 (20) |
| Serum Ferritin, ng/mL | 1058.38 ± 980.58 |
Characteristics of Patients
Overall, 56 (80%) cases had major beta-thalassemia, while 14 (20%) had intermedia beta-thalassemia. Blood group type was O in 24 (34.3%), B in 21 (30%), A in 20 (28.6%), and AB in 5 (7%) participants. A history of splenectomy was recorded in 43 (61.4%) cases. Overall, three (4.3%) cases had a history of close contact with patients with COVID-19 and a positive PCR result. In contrast, three had only positive history of close contact without positive PCR. Generally, the most common comorbidity in the studied population was diabetes mellitus (10%).
The blood samples of all the patients were checked for serological status. The frequency of IgG and IgM positivity was 35.7% (25/70) and 20% (14/70), respectively. In addition, 42.9% (30/70) of the cases were either IgM- or IgG-positive. In the positive and negative IgG groups, the mean age was 27.36 (SD: 9.49) and 23.44 (SD:8.63) years, respectively (P > 0.05). In the positive and negative IgM groups, the mean age was 26.57 (SD: 8.60) and 24.41 (SD: 9.22) years, respectively (P > 0.05). In the positive IgG/positive IgM group, the mean age was 27.80 (SD: 9.12) years, while it was 22.62 (SD: 8.50) in the negative IgG/negative IgM group (P = 0.017).
In this study, 16 (53%) and 18 (60%) cases were female in the positive and negative groups, respectively (P > 0.05).
4.1. Clinical Findings
Among the serologically positive participants, 6 patients had at least one of the symptoms suspected of being infected with COVID-19. Of these six patients, one had a fever, two had a cough, one had anosmia, one had dyspnea, and one had both cough and anosmia. Only one of the patients was hospitalized (1%) with fever and dyspnea. No Intensive Care Unit (ICU) admission or mortality was reported in our study. Three of 30 serologically positive cases (10%) and 3 of 40 serologically negative cases (7%) had exposure to confirmed cases of COVID-19. In serologically positive and negative patients, 24 (80%) and 19 (47.5%) cases had a history of splenectomy, respectively. Thus, the frequency of splenectomy was significantly higher in patients with positive antibodies against COVID-19 compared to the group with negative antibodies (P = 0.005).
5. Discussion
In this study, 57.1% of all participants were serologically positive for COVID-19, while 20% of positive cases had a history of clinical manifestations compatible with COVID-19. Only one of the patients was hospitalized secondary to COVID-19, and no mortality was reported. Therefore, it seems that COVID-19 is more likely to cause minimal or no symptoms in thalassemia cases. Several studies evaluated the immunologic status of patients with beta-thalassemia against COVID-19. In a systematic review by Zafari et al. (8), patients with beta-thalassemia dependent on the transfusion were included, 64.2% of whom were hospitalized following COVID-19, and 42.8% of the deceased. However, our results showed only a 1% history of hospitalization and no mortality. This may be due to the difference in the populations assessed in the systematic review. The age range of the participants in the systematic review was 17-66 years, while our study evaluated the pediatric and young adult populations.
Another study by Sotiriou et al. (14) on patients with beta-thalassemia showed that increasing age was associated with severe clinical presentations, ICU admission, and COVID-19-associated mortality in these patients. In our study, we had only 1% of severe clinical presentations and hospital admission. It is suggested that the effect of increasing age on the clinical presentations of COVID-19 be evaluated within future studies with a larger sample size. In another review study by Hashemieh and Shirvani (15), the patients with beta-thalassemia showed a higher risk of severe clinical events, which was contrary to the results of our study that showed rare severe clinical presentations in patients with beta-thalassemia and COVID-19.
Another nationwide study in Iran on patients with beta-thalassemia by Karimi et al. (12) indicated that the overall mortality rate of thalassemia patients with COVID-19 was 18.6%, which was higher than normal Iranian population at that time (4.71%). The mortality rate in our study was 0% which was lower than the 6.2 - 4.2% COVID-19 mortality rate in the normal population at that time in Iran (16). Comparing the results of studies by Karimi et al. (12) and Sotiriou et al. (14), and our study, we found that the pediatrics and young adults with beta-thalassemia and COVID-19 showed no severe clinical presentations, while the adult thalassemia patients presented with more severe presentations which even led to ICU admission and death in some cases. In the study by Karimi et al., the mean age of patients was 35.3 ± 11.5 years (range: 9 - 67 years), while in our study, the mean age was 24.84 ± 9.08 years, which was lower than the mentioned study.
In concordance with our study, the results of Mahmoudi et al. (17) and Pokorska-Spiewak et al. (18) showed a lower severity of COVID-19 clinical course in children than adults. Therefore, this difference in clinical presentations between pediatrics and adults with beta-thalassemia affected by COVID-19 may be justified by the underlying presence of less severe COVID-19-related clinical presentations in children than in adults.
Few reports have been published about COVID-19 antibodies in patients with beta-thalassemia. A study by Lansiaux et al. (19) showed immunity against COVID-19 in patients with heterozygote beta-thalassemia after evaluating their COVID-19-related antibodies in three Italian regions. In this study, the most common concurrent disease with beta-thalassemia was diabetes, similar to our study. In that survey, beta-thalassemia patients could develop immunity against
COVID-19 as beta chain, the potential target of this virus, could be either absent or less prominent in these cases.
In this study, the frequency of splenectomy was significantly higher in patients with positive antibodies against COVID-19 compared to the group with negative antibodies. Karimi et al. and Motta et al. demonstrated that splenectomy did not affect the clinical course of COVID-19 (12, 13). Moreover, the blood group was not significantly different between groups. However, in another study, the association between blood group and risk of COVID-19 was shown, and blood group A was at a higher risk of infection. In contrast, blood group O was protective against the infection (20, 21).
Our study had some limitations. The sample size was small, and it is suggested to re-evaluate these outcomes within future studies with a larger sample size. In addition, we did not assess the difference between COVID-19 antibodies and clinical presentations based on the transfusion dependency of patients, which should be assessed in future studies. Moreover, some variables of this study were assessed according to checklists and were based on the memory of patients or their parents, which might affect the accuracy of information. The study was retrospective, and during that time, there were not enough facilities for PCR testing in Iran.
5.1. Conclusions
According to the results of this study, it seems that COVID-19 in patients with underlying major and intermedia beta-thalassemia who need blood transfusion might not have severe clinical presentations and mortality.
5.2. Declarations
Acknowledgments: We thank Ali Asghar Clinical Research Development Center (AACRDC) for their assistance in recruiting eligible subjects and funding support.