1. Background
Nephrotic syndrome (NS) is the most common kidney disease in children, affecting 1 to 17 out of every 100,000 children annually (1). In children, the diagnostic criteria for NS include edema, hypoalbuminemia (less than 3 g/dL), proteinuria (more than 40 mg/m2/h), and hypercholesterolemia (more than 250 mg/dL) (2, 3). Nephrotic syndrome is transmitted by both genetic and environmental factors (3, 4). Additionally, immune system dysfunction that destroys the renal filtration barrier could cause NS. Tumor necrosis factor (TNF) and transforming growth factor (TGF) are the starting points for NS and are progressed by the deposition of immune complexes and autoantibodies against the basement membrane. Overactivity of T-cells (CD8 and T helper 1 and T helper 2) is conducted to increase levels of inflammatory cytokines (interleukin 1, IL4, 2, 12, 18) (5).
Several diseases are associated with NS, but minimal-change nephrotic syndrome (MCNS) is the most common type (6). It is still difficult to draw a conclusion about NS prognosis and clinical consequences. Every NS case received empiric corticosteroid therapy due to the 90 - 95% treatment response rate for MCNS, the most common type of NS in children (7). Nephrogenesis occurs in utero between 34 and 36 weeks of gestation. Sixty percent of nephrons promote during the third trimester, putting premature infants at significant risk for inadequate kidney growth. An adjusting reaction to increasing distinct nephron glomerular filtration rate occurs when the number of nephrons decreases in low birth weight (LBW), resulting in decreased glomerular filtration surface area. Early-onset renal disease is brought on by glomerular filtration abnormalities and damaged podocytes (8). This reaction then raises the risk of developing kidney damage and type of illness (9, 10). Hypertension (11), obesity (12), renal disease (13), cardiovascular disease (14), and other chronic diseases are all linked to LBW. Several studies have shown that patients with LBW have more relapses and steroid-resistant nephrotic syndrome (SRNS) (8). According to previous studies investigating the effect of birth weight on the consequences of NS, the response to a corticosteroid regimen in NS is the most important prognostic factor. These studies also found that children with higher rates of LBW had an exacerbated course of NS (14), which, in some studies, was lower in the LBW group than in the group with normal birth weight (NBW).
2. Objectives
The present study was designed because NS has many causes, including genetic and environmental etiologies, and according to contradictory studies on the effect of LBW on the incidence of NS and treatment response, as well as the frequency of LBW babies in Sistan and Balouchestan province.
3. Methods
Two hundred and ninety-two children with NS aged 1 to 18 years were considered for the present cross-section study. The children were followed up at Ali-Ibn-e-Abitaleb Hospital in Zahedan, Iran, from 2010 to 2021. Some of these profiles were excluded from the study due to missing data based on their mother’s reports. Children with nephrotic conditions at birth, a disease affecting multiple organs, or other causes of NS were also excluded. The missing data were related to birth weight, diagnosis date, or gestational age (total of 33 children). After excluding these children from the study, 259 were analyzed.
Nephrotic syndrome is characterized by nephrotic-range proteinuria and either hypoalbuminemia (serum albumin < 30 g/L) or edema when serum albumin level is not available (1). Complete remission within 4 weeks of prednisone or prednisolone at the standard dose (60 mg/m2/day or 2 mg/kg/day, maximum 60 mg/day) is known as steroid-sensitive nephrotic syndrome (SSNS). Lack of complete remission within four weeks of treatment with prednisolone at standard dose is called SRNS (2). We defined relapse as having less than 3.0 g/L of protein in the urine for three consecutive days, necessitating the use of steroids, which led to an increase in the dose. The definition of frequently relapsing nephrotic syndrome (FRNS) was less than two relapses within six months of the disease or less than four relapses in a given year. The results of paternal self-reported questionnaires regarding birth-related information were accessed via medical chart review. The World Health Organization (WHO) defines LBW as a child born weighing less than 2500 g. As a result, children were deliberately given an NBW if they were born weighing 2500 g. The present research was approved (code: IR.ZAUMS.REC.1399.377) by the Ethics Committee of the Faculty of Medicine at Zahedan University.
3.1. Statistical Analysis
The subjects' data are presented in the form of a mean, standard deviation (SD), or proportions (repeatability of group findings). The student t-test or the chi-square test is used for statistical analysis. Binary logistic regression was used to explain the relationship between treatment response and independent variables. At P = 0.05, differences are considered statistically significant.
4. Results
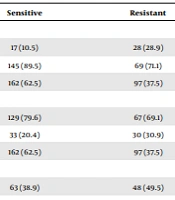
We identified 292 children with NS between 2010 and 2021. Of them, 259 meeting the inclusion criteria were registered. For the study duration, each patient received a single dose of corticosteroid (prednisone or prednisolone) at a concentration of two milligrams per kilogram (maximum sixty milligrams) once every four to six weeks for a total of six weeks. A total of 259 children participating in this study were divided into two groups: Steroid-sensitive (n = 162) and steroid-resistant (n = 97). Steroid-sensitive nephrotic syndrome is more common in children than SRNS (62.5% vs. 37.5%). The subjects’ mean age at disease was 5.35 ± 2.89 years in the steroid-sensitive group and 6.18 ± 3.67 years in the steroid-resistant group (P = 0.46). Comparing the steroid-sensitive group with the steroid-resistant group indicated that the birth weight was 3.0 ± 0.54 versus 2.77 ± 0.66, respectively (P = 0.005). The two groups' serum levels of cholesterol, triglycerides, albumin, and creatinine were not significantly different. Additionally, the steroid-resistant group had a more significant frequency number than the steroid-sensitive group (1.06 ± 0.65 versus 1.91 ± 0.83; P = 0.001) (Table 1). The study population consisted of 51.7% males and 42.9% females. Low birth weight (< 2500 g) was significantly higher in the steroid-resistant than in the steroid-sensitive group (P = 0.001) (Table 2). Birth weight (P = 0.04, OR = 0.58, 95% CI: 0.34 - 0.99) and number of recurrences (P = 0.001, OR = 4.60, 95% CI: 2.91 - 7.27) were both found to be significantly associated with the development of steroid resistance (Table 3).
| Parameters | All Children | Steroid Responsiveness Status of Nephrotic Syndrome | Significance Level | |
|---|---|---|---|---|
| Sensitive | Resistant | |||
| Age at diagnosis (y) | 5.66 ± 3.22 | 5.35 ± 2.89 | 6.18 ± 3.67 | 0.46 |
| Birth weight (kg) | 2.92 ± 0.60 | 3.00 ± 0.54 | 2.77 ± 0.66 | 0.005 b |
| Serum triglyceride, mg/dL | 234.09 ± 122.04 | 223.4 ± 110.64 | 251.93 ± 137.77 | 0.085 |
| Serum cholesterol, mg/dL | 362.87 ± 112.64 | 360.32 ± 110.48 | 367.13 ± 116.60 | 0.63 |
| Serum albumin, mg/dL | 2.40 ± 0.57 | 2.43 ± 0.59 | 2.35 ± 0.54 | 0.27 |
| Serum creatinine, mg/dL | 0.72 ± 0.78 | 0.65 ± 0.61 | 0.84 ± 1.0 | 0.092 |
| Weight at diagnosis (kg) | 19.45 ± 8.47 | 18.55 ± 7.40 | 20.96 ± 9.86 | 0.38 |
| Number of recurrences | 1.38 ± 0.83 | 1.06 ± 0.65 | 1.91 ± 0.83 | 0.0001 b |
Abbreviation: SD, standard deviation.
a Values are expressed as mean ± SD unless otherwise indicated.
b P-value < 0.05 is significant.
| Parameters | Steroid Responsiveness Status of Nephrotic Syndrome | Significance Level | ||
|---|---|---|---|---|
| Sensitive | Resistant | Total | ||
| Birth weight (kg) | 0.001 b | |||
| Low birth weight | 17 (10.5) | 28 (28.9) | 45 (17.4) | |
| Normal birth weight | 145 (89.5) | 69 (71.1) | 214 (82.6) | |
| Total | 162 (62.5) | 97 (37.5) | 259 (100) | |
| Age at diagnosis | 0.039 b | |||
| Age < 8 | 129 (79.6) | 67 (69.1) | 196 (75.5) | |
| Age > 8 | 33 (20.4) | 30 (30.9) | 63 (24.3) | |
| Total | 162 (62.5) | 97 (37.5) | 259 (100) | |
| Sex | 0.095 | |||
| Female | 63 (38.9) | 48 (49.5) | 111 (42.9) | |
| Male | 99 (61.1) | 49 (50.5) | 148 (51.1) | |
| Total | 162 (62.5) | 97 (37.5) | 259 (100) | |
a Values are expressed as No. (%) unless otherwise indicated.
b P-value < 0.05 is significant.
5. Discussion
The most prevalent disease in children is NS, which can progress to severe forms that necessitate frequent recurrences and require long-term corticosteroid treatment at high doses (1). In our study, SSNS is more common in children than SRNS (62.5% vs. 37.5%). Birth weight was higher in steroid-sensitive than in steroid-resistant children (3.0 ± 0.54 versus 2.77 ± 0.66; P = 0.005). According to recent epidemiologic studies, there is a strong correlation between LBW and NS. In these studies, such as Conti et al. (15), steroid resistance risk was found to be significantly linked to LBW, similar to the current study.
Prematurity and LBW were identified as risk factors by Ikezumi et al. (9), suggesting that LBW is more common in children with NS than in healthy children. Sixteen patients had Focal segmental glomerulosclerosis (FSGS), of which 6 (37.5%) had LBW; this LBW rate was significantly higher than the overall LBW rate in Japan (9.7%). The incidence of LBW was also high in patients with MCNS (12.5%). The majority of patients with MCNS underwent biopsies due to their unfavorable clinical course, which included a significantly high rate of relapse or high reliance on steroid treatment. As a result, the MCNS group had a relatively high incidence of LBW (12.5%), and the findings of other studies suggested that LBW might also be a risk factor for a refractory NS. Although we did not have a control group, these findings are in line with our findings. Teeninga et al. (16) also looked into the possibility of recurrence and steroid resistance in MCNS patients and discovered a significant correlation between LBW and an increased risk of relapse and steroid resistance in patients with LBW. There were 201 MCNS patients investigated in this study, with 176 having an NBW and 25 being underweight. In addition, they demonstrated that individuals with lower weights were significantly more likely to require cytotoxic medications and to develop steroid resistance. However, in accordance with our findings, the LBW group had a higher one-year recurrence rate than the standard birth weight group. Furthermore, Rezavand et al. (17) looked into NS children referring to Imam Reza Hospital to examine the connection between LBW and the likelihood of NS in children and discovered that patients with LBW had twice the risk of developing NS as those with NBW. However, there was no statistically significant difference. Even though their study revealed no statistically significant relationship between NS and LBW, it did reveal that the risk of NS was twice as high in the case group as in the control group. This study was conducted in Iran, the same nation as ours, which may suggest that ethnicity and genetic diversity play a role in the prognostic factors of NS. However, another study in Iran on 54 male and 23 female patients indicated that premature birth did not appear to be associated with the number of recurrences (P-value = 0.99). The birth weight of patients who recurred less than twice and those who recurred more than twice in six months was not significantly different (P = 0.336) using the Mann-Whitney U test. In addition, Fisher's exact test revealed no significant association (P-value = 0.643) between the likelihood of developing steroid resistance and premature birth. In addition, there was no statistically significant relationship between birth weight and steroid resistance (P-value = 0.768) (18), which was not consistent with our findings. Konstantelos et al.’s (8) study investigated 336 children and adolescents with NS, and steroid resistance was shown to be about 3.16 times more likely to occur in LBW children than in NBW children. Our findings are also supported by the mentioned study's findings. As mentioned previously, NS may be caused by a variety of other factors. As a result, it is recommended that additional research is conducted using larger samples from various ethnic groups. Research has found a connection between the disease course and birth weight and weight gain during the first two years of life. In Plank et al.’s study, there was a higher proportion of those with steroid resistance compared to the children who were considered appropriate for gestational age (AGA), and arterial hypertension exacerbated the disease course (19). Na et al. reviewed the medical records of 56 Korean children with NS and showed that steroid resistance was significantly more prevalent in the small for gestational age (SGA) group in their study (14). Multiple studies have demonstrated that children of varying birth weights have distinct steroid responses, which are consistent with our findings regarding SRNS. The parental self-reported birth weights are one of the present study's limitations. Nevertheless, studies have shown that the accuracy of parental self-reported birth weights can be used for both clinical and epidemiological purposes (14, 19).
5.1. Conclusions
We have shown that LBW harms the course and prognosis of NS in children such that children with LBW have more resistance to treatment and more recurrence. These findings help clinicians and parents in assessing the expected clinical practices. Furthermore, as an additional pathogenic principal in renal disease, perinatal programming needs further investigation.